Braun Aesculap S4 Manual
Other Braun Medical Equipment manuals

Braun
Braun Infusomat P User manual

Braun
Braun Aesculap Acculan 4 Manual
Braun
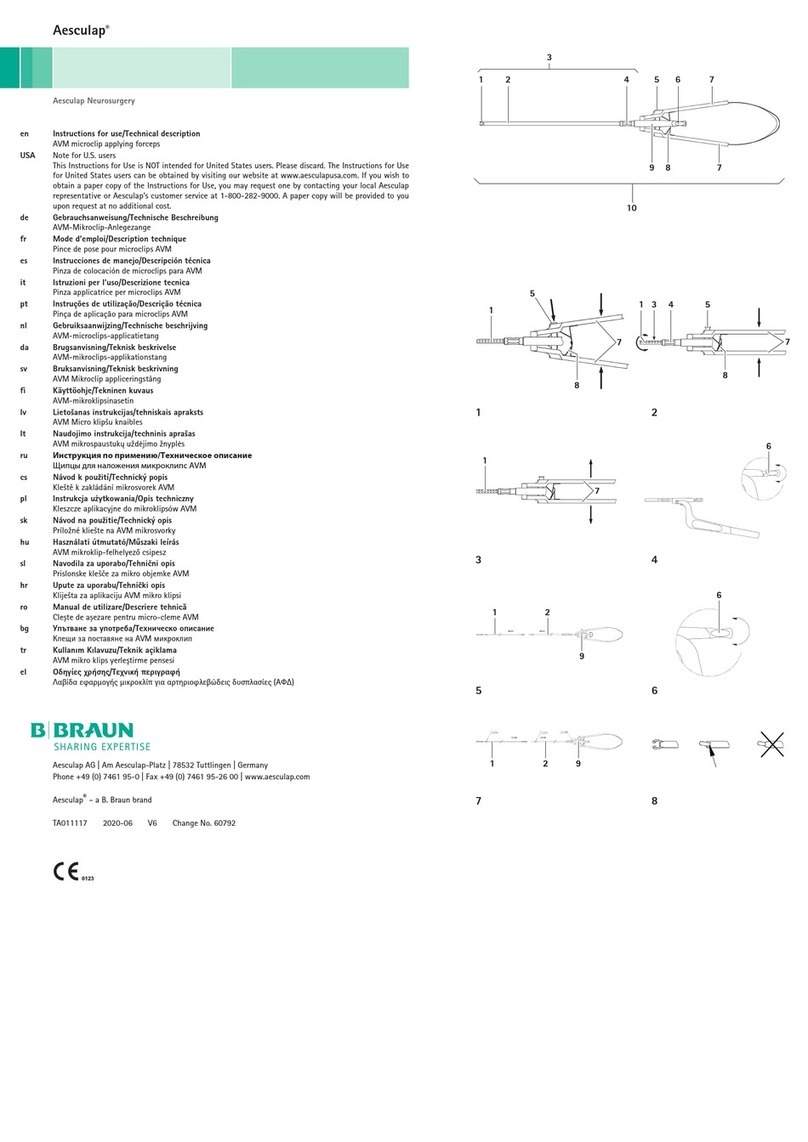
Braun Aesculap Neurosurgery Manual

Braun
Braun Aesculap Acculan 4 Manual
Braun
Braun BPX800 User manual
Braun
Braun Aesculap ELAN 4 Manual
Braun
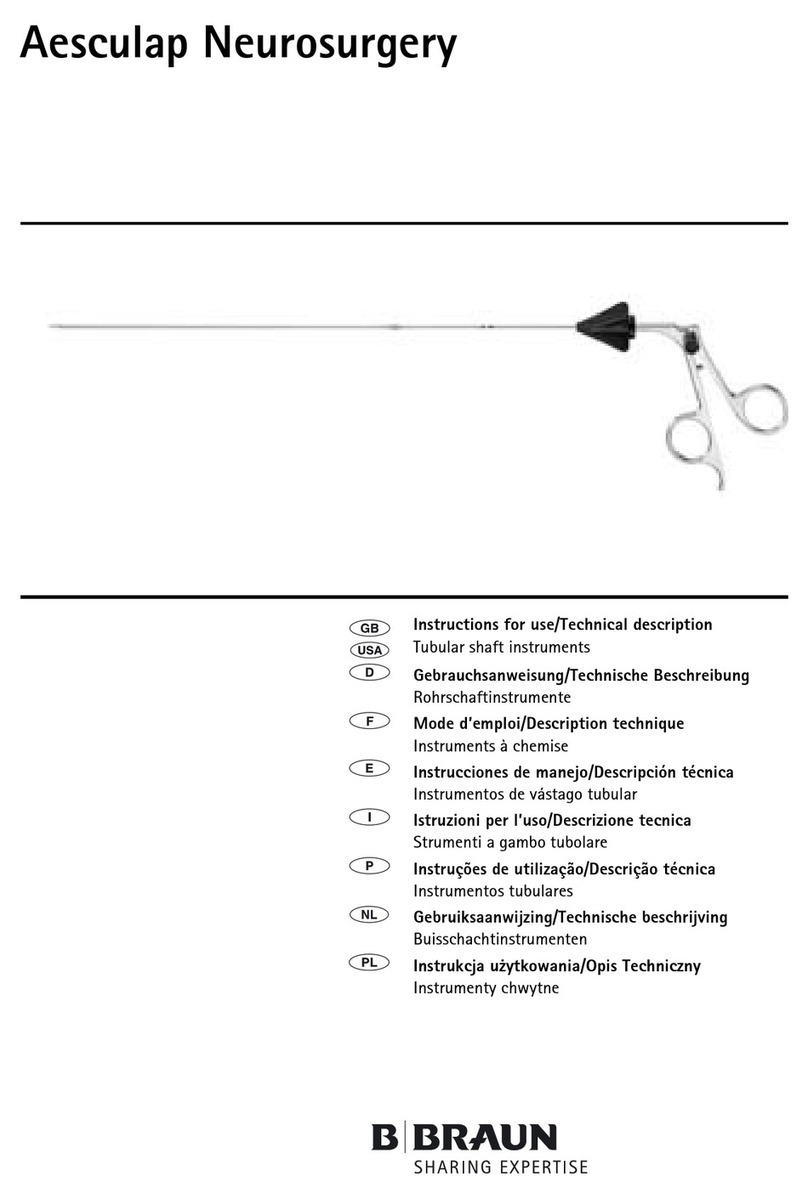
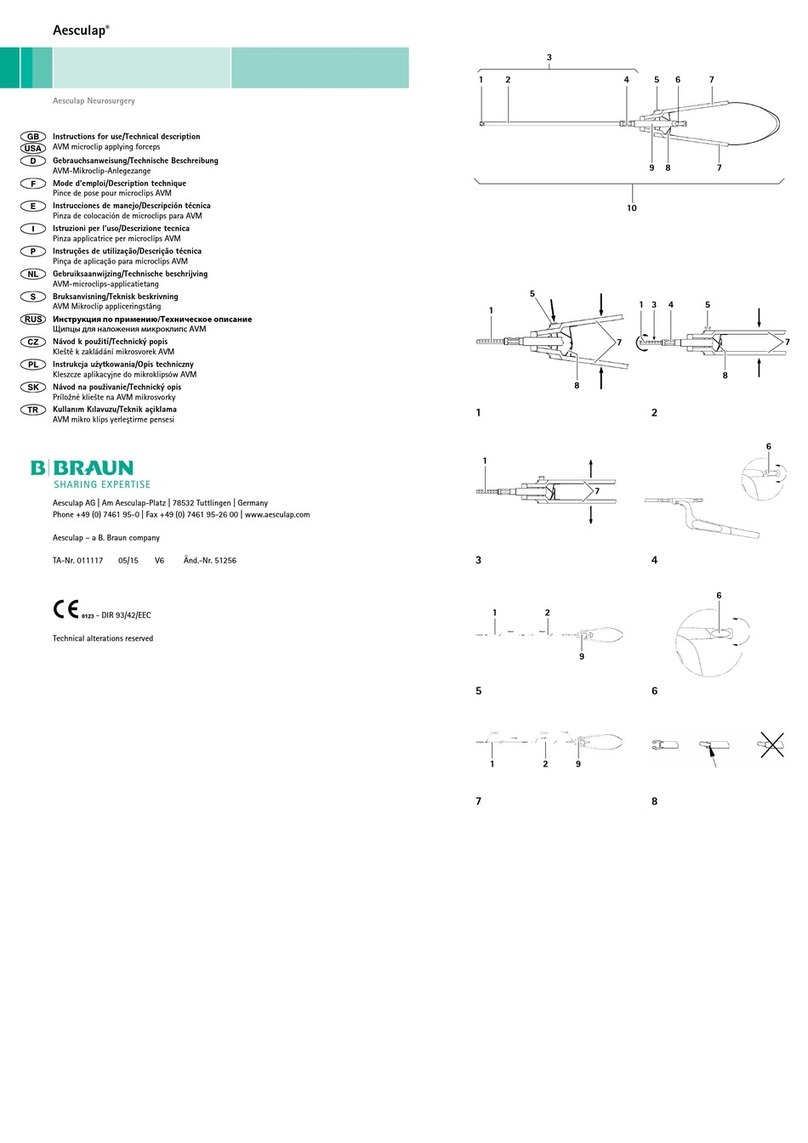
Braun Aesculap Neurosurgery Manual

Braun
Braun Acculan 3Ti User manual
Braun
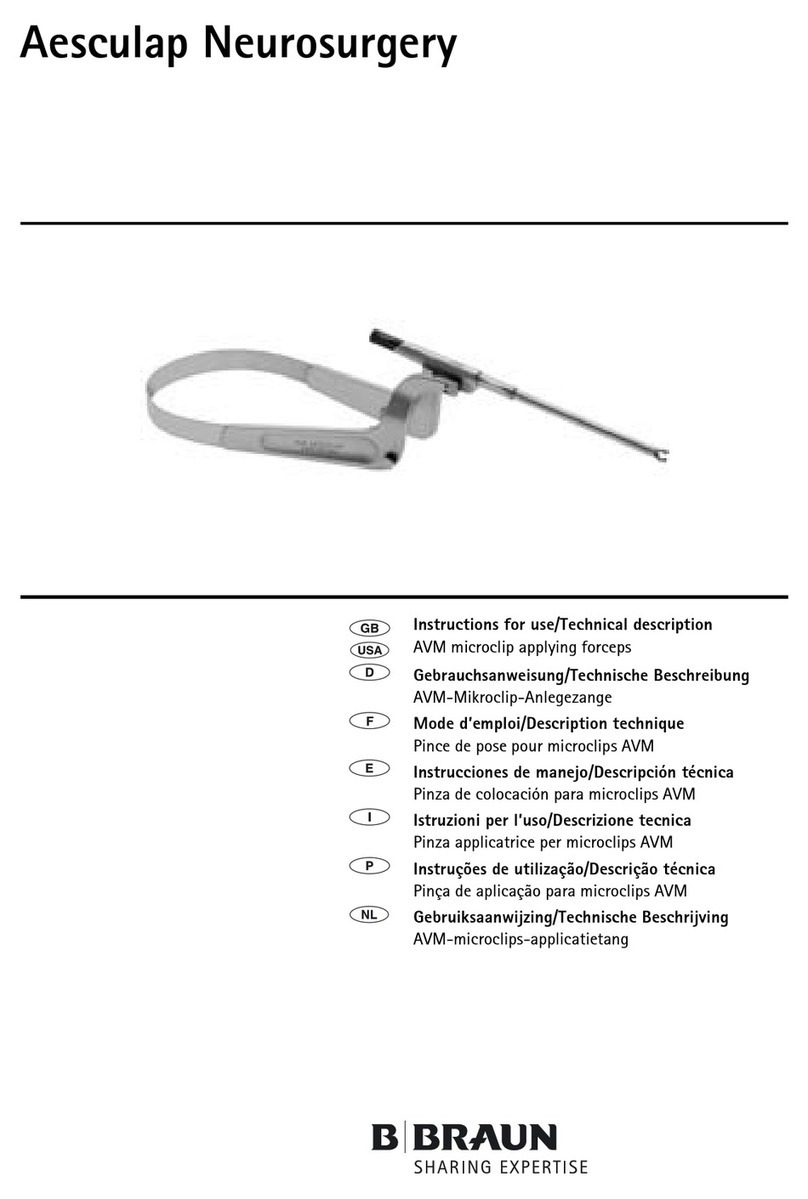
Braun Aesculap Neurosurgery Manual

Braun
Braun Draina S Large User manual
Braun
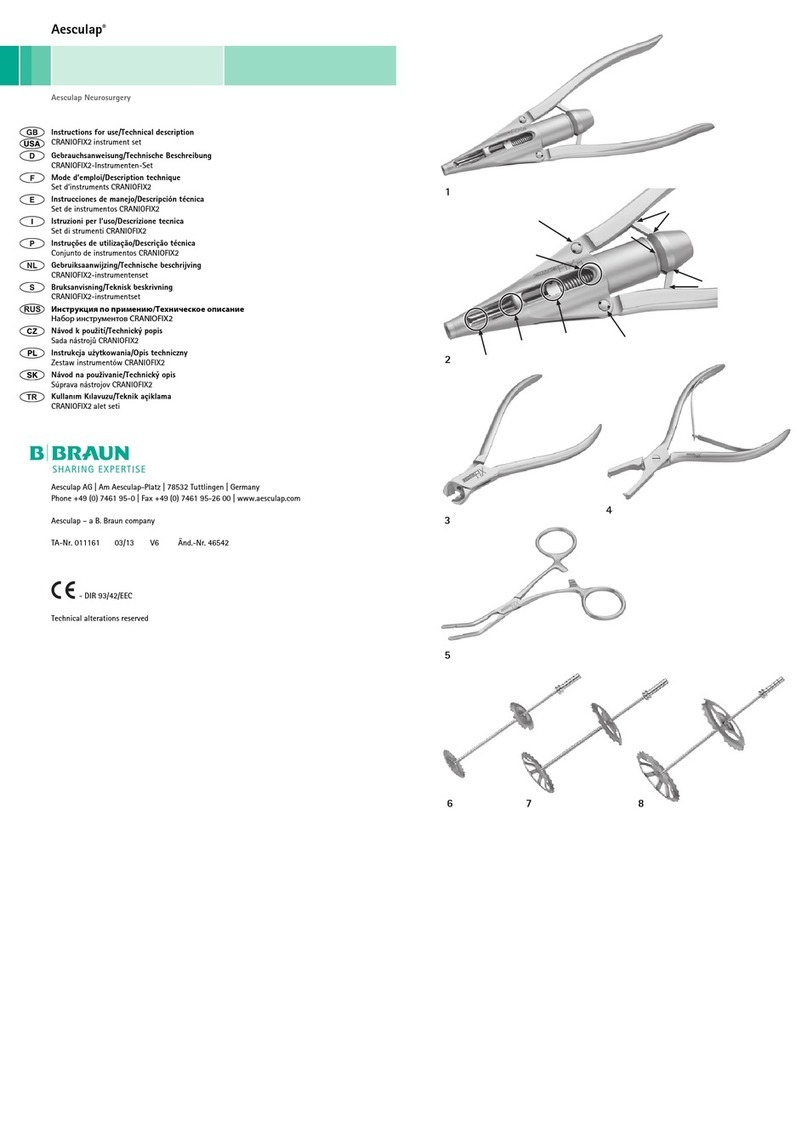
Braun Aesculap Neurosurgery Manual

Braun
Braun Infusomat Space P User manual

Braun
Braun Aesculap activ L User manual

Braun
Braun Aesculap S4 FW225R Manual

Braun
Braun Aesculap Spine User manual

Braun
Braun Perfusor compact plus User manual

Braun
Braun Flexima 3S User manual

Braun
Braun Infusomat Space User manual
Braun
Braun BNA100US User manual

Braun
Braun Aesculap Quintex Manual