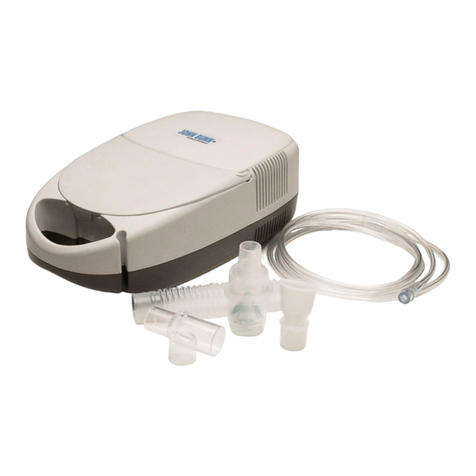
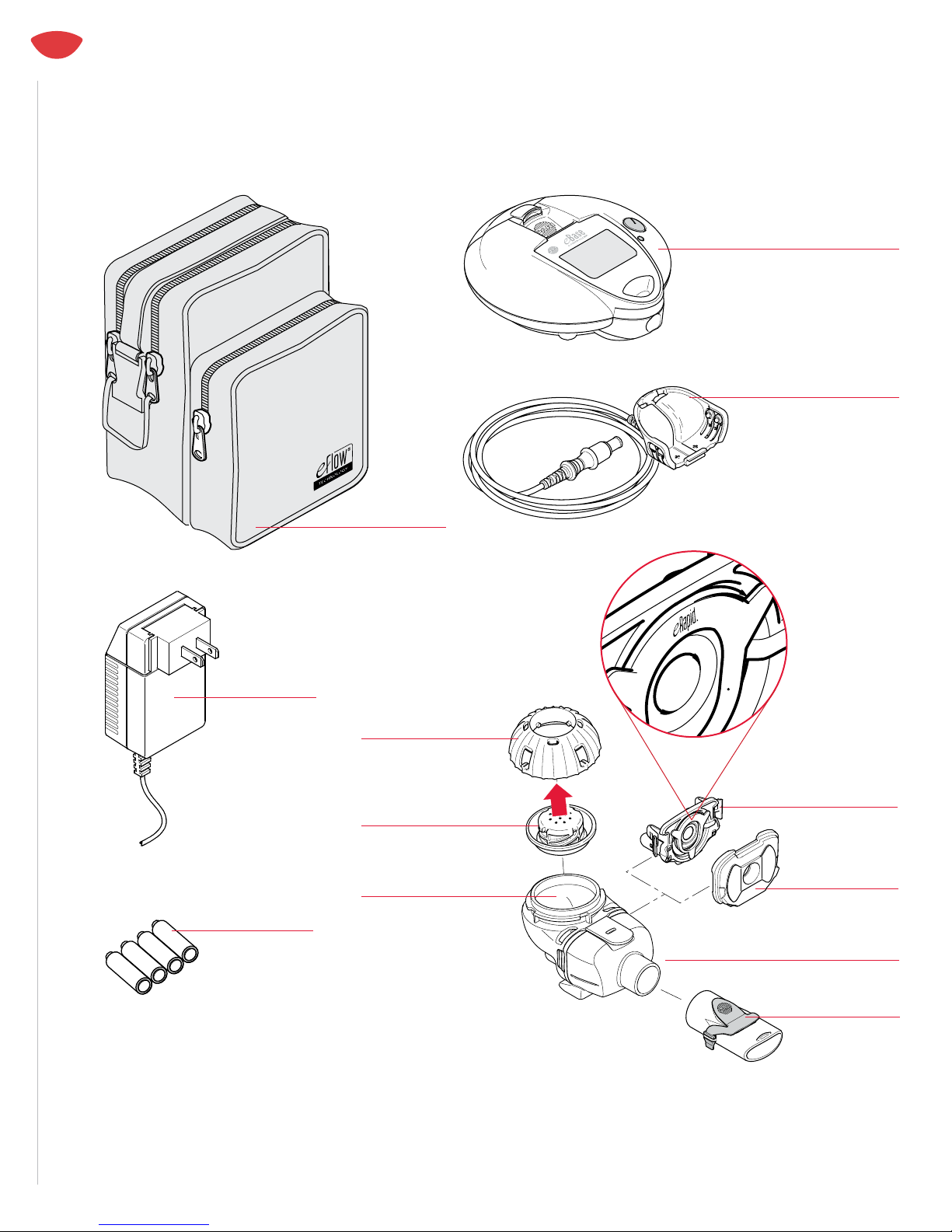
QUESTIONS ABOUT ERAPID? CALL 1-866-335-6943 | 10
GTAKING A TREATMENT
1. Make sure the eRapid Nebulizer Handset is on a at,
stable surface.
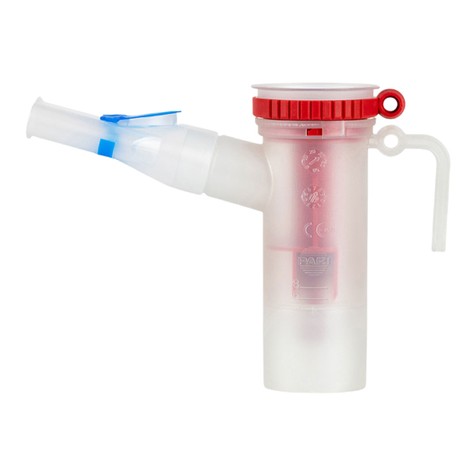
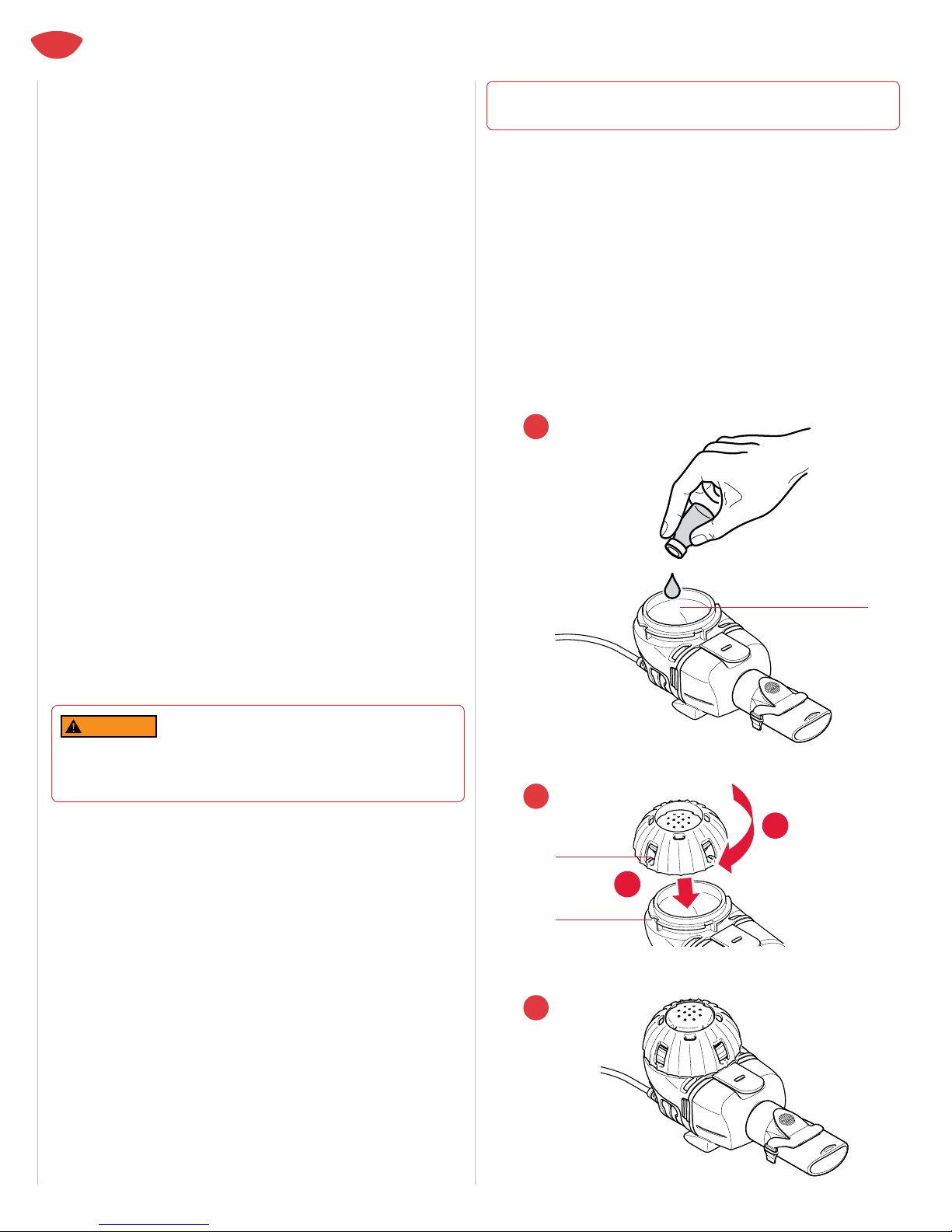
2. Pour only the medication prescribed by your physician
specically for the eRapid Nebulizer System into the eRapid
Nebulizer Handset Medication Reservoir (Figure 13). DO NOT
USE MEDICATIONS PRESCRIBED FOR OTHER NEBULIZERS IN THE
ERAPID NEBULIZER SYSTEM. NOTE: THE MAXIMUM VOLUME FILL
IS 6 ML. DO NOT OVERFILL THE MEDICATION RESERVOIR.
3. Align the Tabs on the Medication Cap with the Tab Slots on the
Medication Reservoir (Figure 14a). Turn the Medication Cap
clockwise until it stops (Figure 14b). Your eRapid Nebulizer Handset
is now ready, and you can begin your treatment (Figure 15).
4. To begin your treatment, sit in a relaxed, upright position. Press
and hold the On/O button for a few seconds (Figure 16). You
will hear one “beep” and the status light will illuminate green.
The eFlow Technology logo will appear. This is the START screen.
(Figures 16-17c). The Controller is now ON.
5. After a few seconds, aerosol mist will begin to ow into the
Aerosol Chamber of the eRapid Nebulizer Handset.
6. Your Controller is designed to display the TREATMENT screen
(Figure 17b) if the eRapid Nebulizer System is assembled
correctly and working properly.
7. Place the Mouthpiece on top of your bottom lip and tongue and
close your lips around it. Hold the eRapid Nebulizer Handset level.
If held down more than 45 degrees, the Aerosol Head will emit
2 beeps and the status light on the Controller will ash 2 green
lights. After approximately 30 seconds the Controller will shut o.
If this occurs, hold the eRapid Nebulizer Handset level and press
the Controller’s On/O Button to resume the treatment.
WARNING
Keep the eRapid Nebulizer Handset level when in use. If the
handset is tilted, the dosage may not be accurate.
8. Breath normally in and out through the Mouthpiece. Continue to
inhale and exhale comfortably until the treatment is nished.
9. When all of the medication has been delivered you will hear
the Aerosol Head emit 2 beeps, the DOSE COMPLETE screen will
appear (Figure 18), and the Controller will automatically shut o.
10. The eBase Controller included with the eRapid includes software
that allows a PAUSE, when necessary. If for any reason you
must PAUSE in your treatment before its completion, press
and hold the Controller’s On/O Button for one full second. The
eRapid’s PAUSE mode is activated when the LED ashes GREEN
and your Controller display switches from the TREATMENT
screen to the PAUSE screen (Figure 17c). When you are ready
to resume your treatment, press and hold the Controller On/O
Button for one full second. The treatment will then resume, with
the Controller display switching from the PAUSE screen to the
TREATMENT screen.
Figure 13
Figure 14
Figure 15
MEDICATION RESERVOIR
a
b
TAB
TAB SLOT
NOTE: eBase Controllers supplied with other eFlow Technology
devices may not have this software feature.
11. The Controller will shut o automatically at the end of the
treatment. Unique to the eRapid Nebulizer Handset design is
about 1mL (or 1/5 teaspoon) of residual volume of medication
which mimics other general purpose nebulizers intended to
deliver approved medications. Open the Medication Cap and check
to make sure that there is not more than 1 mL of medication
following a complete treatment. If there is, replace the Medication
Cap and resume the treatment until only about 1mL remains in
the Medication Reservoir (Fig. 19).
12. Once the treatment is complete, disassemble the eRapid
Nebulizer Handset for cleaning. Refer to Section H for
cleaning instructions.