ITALIANO
INDICE
Illustrazioni ..............................................................
11-15
1 DESCRIZIONE DEL DISPOSITIVO .....................................60
1.1 Branca iliaca per endoprotesi addominale Zenith Spiral-Z ............ 60
1.2 Sistema di inserimento............................................... 60
2 INDICAZIONI PER L’USO.............................................60
3 CONTROINDICAZIONI...............................................60
4 AVVERTENZE E PRECAUZIONI .......................................60
4.1 Informazioni generali ................................................ 60
4.2 Selezione, trattamento e follow-up del paziente...................... 60
4.3 Tecniche preoperatorie di misurazione e imaging .................... 60
4.4 Selezione del dispositivo............................................. 61
4.5 Procedura di impianto ............................................... 61
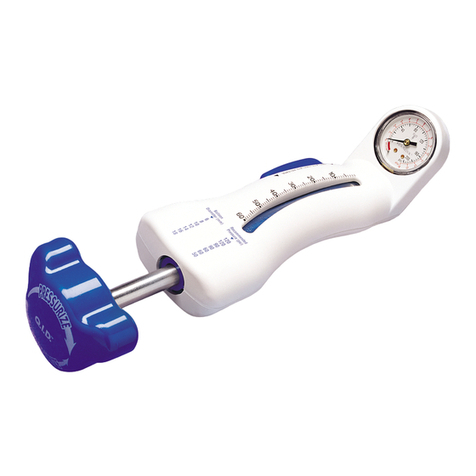
4.6 Uso del palloncino dilatatore......................................... 61
4.7 Informazioni sulle procedure MRI .................................... 61
Campo magnetico statico ........................................... 61
Riscaldamento correlato all’MRI ..................................... 61
Artefatti d’immagine ................................................ 61
5 EVENTI NEGATIVI ...................................................61
5.1 Eventi negativi osservati ............................................. 61
5.2 Possibili eventi negativi .............................................. 61
Notifica degli eventi negativi correlati al dispositivo ................. 62
6 RIEPILOGO DEGLI STUDI CLINICI.....................................62
7 SELEZIONE E TRATTAMENTO DEI PAZIENTI ...........................62
7.1 Requisiti per il trattamento........................................... 62
8 INFORMAZIONI DA FORNIRE AI PAZIENTI ............................62
9 CONFEZIONAMENTO. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .62
10 INFORMAZIONI PER USO CLINICO ..................................62
10.1 Programma di formazione per il medico ............................ 62
10.2 Esame prima dell’uso ............................................... 63
10.3 Materiali necessari.................................................. 63
10.4 Materiali consigliati................................................. 63
10.5 Linee guida per la determinazione del diametro idoneo
del dispositivo........................................................... 63
Tabella 10.5.1 Guida alla determinazione delle dimensioni idonee
della branca iliaca per endoprotesi addominale Spiral-Z ............. 63
11 ISTRUZIONI PER L’USO.............................................63
Requisiti anatomici.................................................. 63
Informazioni generali sull’impiego................................... 63
Fattori da considerare in sede preliminare ........................... 63
Preparazione del paziente........................................... 63
11.1 Sistema con branca iliaca per endoprotesi addominale
Zenith Spiral-Z .......................................................... 63
11.1.1 Preparazione/lavaggio della branca iliaca controlaterale....... 63
11.1.2 Preparazione/lavaggio della branca iliaca ipsilaterale.......... 63
11.1.3 Accesso vascolare e angiografia............................... 63
11.1.4 Posizionamento e rilascio della branca iliaca controlaterale.... 63
11.1.5 Posizionamento e rilascio della branca iliaca ipsilaterale ....... 64
11.1.6 Inserimento del palloncino dilatatore ......................... 64
Angiogramma conclusivo ........................................... 64
12 LINEE GUIDA PER LE TECNICHE DI IMAGING E IL FOLLOW-UP
POSTOPERATORIO. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .64
12.1 Informazioni generali ............................................... 64
12.2 Ulteriori esami di controllo e trattamento ........................... 64
12.3 Informazioni sulle procedure MRI ................................... 64
Campo magnetico statico. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 64
Riscaldamento correlato all’MRI..................................... 65
Artefatti d’immagine. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 65
13 INFORMAZIONI DOCUMENTANTI IL DISPOSITIVO ...................65
NEDERLANDS
INHOUD
Afbeeldingen............................................................
11-15
1 BESCHRIJVING VAN HET HULPMIDDEL...............................66
1.1 Zenith Spiral-Z AAA iliacale poot ..................................... 66
1.2 Plaatsingssysteem ................................................... 66
2 INDICATIES VOOR GEBRUIK .........................................66
3 CONTRA-INDICATIES................................................66
4 WAARSCHUWINGEN EN VOORZORGSMAATREGELEN .................66
4.1 Algemeen ........................................................... 66
4.2 Selectie, behandeling en controle van de patiënt..................... 66
4.3 Preprocedurele meettechnieken en beeldvorming ................... 66
4.4 Selectie van het hulpmiddel ......................................... 67
4.5 De implantatieprocedure ............................................ 67
4.6 Gebruik van de modelleerballon ..................................... 67
4.7 MRI-informatie....................................................... 67
Statisch magnetisch veld............................................ 67
MRI-gerelateerde opwarming ....................................... 67
Beeldartefact. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 67
5 ONGEWENSTE VOORVALLEN ........................................67
5.1 Waargenomen ongewenste voorvallen............................... 67
5.2 Mogelijke ongewenste voorvallen ................................... 67
Melding van prothesegerelateerde ongewenste voorvallen.......... 68
6 SAMENVATTING VAN KLINISCHE STUDIES............................68
7 SELECTIE EN BEHANDELING VAN DE PATIËNT ........................68
7.1 Individualisering van de behandeling ................................ 68
8 INFORMATIE VOOR DE PATIËNT......................................68
9 WIJZE VAN LEVERING ...............................................68
10 INFORMATIE OVER HET KLINISCHE GEBRUIK ........................68
10.1 Opleiding van de arts............................................... 68
10.2 Inspectie voorafgaand aan gebruik ................................. 68
10.3 Benodigde materialen .............................................. 68
10.4 Aanbevolen materialen............................................. 68
10.5 Leidraad voor het bepalen van de diameter van het hulpmiddel .... 69
Tabel 10.5.1 Spiral-Z AAA iliacale poot – leidraad voor het bepalen
van de maat ........................................................ 69
11 GEBRUIKSAANWIJZING ............................................69
Anatomische vereisten .............................................. 69
Algemene gebruiksinformatie....................................... 69
Bepalende factoren vóór de implantatie............................. 69
Voorbereiding van de patiënt ....................................... 69
11.1 Zenith Spiral-Z AAA iliacaal pootsysteem ........................... 69
11.1.1 Voorbereiding/spoelen van de contralaterale iliacale poot..... 69
11.1.2 Voorbereiding/spoelen van de ipsilaterale iliacale poot........ 69
11.1.3 Vasculaire introductie en angiografie.......................... 69
11.1.4 Plaatsing en ontplooiing van de contralaterale iliacale poot ... 69
11.1.5 Plaatsing en ontplooiing van de ipsilaterale iliacale poot ...... 69
11.1.6 Introductie van de modelleerballon........................... 70
Afrondend angiogram .............................................. 70
12 RICHTLIJNEN VOOR BEELDVORMEND ONDERZOEK EN
POSTOPERATIEVE CONTROLE ......................................70
12.1 Algemeen .......................................................... 70
12.2 Extra controle en behandeling...................................... 70
12.3 MRI-informatie ..................................................... 70
Statisch magnetisch veld ........................................... 70
MRI-gerelateerde opwarming....................................... 70
Beeldartefact ....................................................... 71
13 INFORMATIE PATIËNTVOLGSYSTEEM ...............................71