13I-ZDEG-EU-1312-394-03
ENGLISH
ZENITH® TX2® DISSECTION ENDOVASCULAR
GRAFT WITH PROFORM® AND THE ZTRAK® PLUS
INTRODUCTION SYSTEM
Read all instructions carefully. Failure to properly follow the instructions,
warnings, and precautions may lead to serious consequences or injury to
the patient.
CAUTION: Federal (U.S.A.) law restricts this device to sale by or on the order
of a physician.
CAUTION: All contents of the inner pouch (including the introduction
system and endovascular graft) are supplied sterile, for single use only.
1 DEVICE DESCRIPTION
1.1 Zenith TX2 Dissection Endovascular Graft with Pro-Form and
the Z-Trak Plus Introduction System
When treating dissections of the descending thoracic aorta, the Zenith TX2
Dissection Endovascular Graft with Pro-Form and the Z-Trak Plus Introduction
System is typically used in conjunction with the Zenith® Dissection Endovascular
Stent. The Zenith TX2 Dissection Endovascular Graft with Pro-Form and the
Z-Trak Plus Introduction System is for sealing of entry tear(s). The Zenith
Dissecton Endovascular Stent provides support to delaminated segments of
the aorta. For information regarding the use and deployment of the Zenith
Dissection Endovascular Stents, please refer to the Instructions for Use.
The Zenith TX2 Dissection Endovascular Graft with Pro-Form and the Z-Trak
Plus Introduction System shortest Straight Components can be used to provide
additional length to the endovascular graft, both proximal and distal. Ensure
there is a minimum overlap of 2 stents. The stent graft is constructed of full-
thickness woven polyester fabric sewn to self-expanding stainless steel Cook-Z
stents with braided polyester and monofilament polypropylene suture (Fig. 1).
The Zenith TX2 Dissection Endovascular Graft with Pro-Form and the Z-Trak Plus
Introduction System is fully stented to provide stability and the expansile force
necessary to open the lumen of the graft during deployment. Additionally, the
Cook-Z stents provide the necessary attachment and seal of the graft to the
vessel wall.
To facilitate fluoroscopic visualization of the stent graft, four radiopaque
markers are positioned on each end of the Straight Component or Tapered
Component. These markers are placed in a circumferential orientation within 1
mm of the most proximal aspect of the graft material and within 1 mm of the
most distal aspect of the graft material.
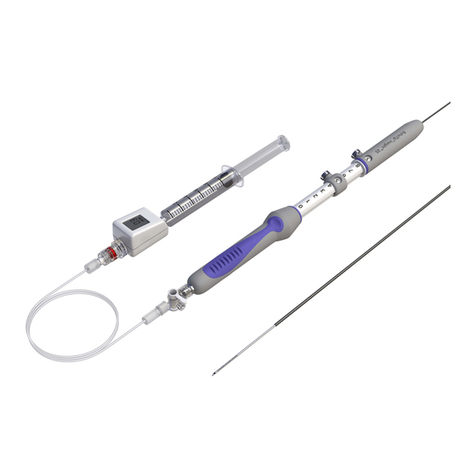
1.2 Thoracic Z-Trak Plus Introduction System
The Zenith TX2 Dissection Endovascular Graft with Pro-Form and the Z-Trak Plus
Introduction System is shipped preloaded onto the Z-Trak Plus Introduction
System. It has a sequential deployment method with built-in features to pro-
vide continuous control of the endovascular graft throughout the deployment
procedure. The Z-Trak Plus Introduction System enables precise positioning
before deployment of the Straight Component or Tapered Component. The
straight and tapered graft components are deployed from a 20 French or 22
French Z-Trak Plus Introduction System. These systems use a single trigger-
wire release mechanism to secure the endovascular graft onto the delivery
introduction system until released by the physician (Fig. 2). All introduction
systems are compatible with a .035 inch wire guide. For added hemostasis, the
Captor® Hemostatic Valve can be loosened or tightened for the introduction
and/or removal of ancillary devices into and out of the sheath. All introduction
systems feature Flexor®introducer sheaths, which resist kinking and are
hydrophilically coated. Both features are intended to enhance trackability in the
iliac arteries and thoracic aorta.
To facilitate sheath withdrawal, each graft component is kept in a longitudinally
stretched condition on the introduction system by locking trigger-wires (Fig. 3).
These trigger-wires work in tandem to deliver sequential controlled release of
the Zenith TX2 Dissection Endovascular Graft with Pro-Form during deployment
(Fig. 3).
2 INTENDED USE
The Zenith TX2 Dissection Endovascular Graft with Pro-Form and the Z-Trak Plus
Introduction System is indicated for the endovascular treatment of patients
with symptomatic dissection of the descending thoracic aorta having vascular
morphology suitable for endovascular repair (Fig. 4), including:
• Adequateiliac/femoralaccesscompatiblewiththerequiredintroduction
systems,
• Radiusofcurvaturegreaterthan35mmalongthelengthofaortaintended
to be treated by Straight or Tapered Component,
• Non-dissected/aneurysmalaorticsegments(fixationsites)proximaltothe
entry tear:
• withalengthofatleast20mm,
• withadiametermeasuredouterwalltoouterwallofnogreaterthan38
mm and no less than 20 mm, and
• withlocalizedangulationlessthan45degrees.
3 CONTRAINDICATIONS
The Zenith TX2 Dissection Endovascular Graft with Pro-Form and with the Z-Trak
Plus Introduction System is contraindicated in:
• Patientswithknownsensitivitiesorallergiestostainless steel, polyester,
polypropylene, nitinol or gold.
• Patientswithasystemicinfectionwhomaybeat increased risk of
endovascular graft infection.
4 WARNINGS AND PRECAUTIONS
4.1 General
• Readallinstructionscarefully.Failuretoproperlyfollowtheinstructions,
warnings, and precautions may lead to serious consequences or injury to the
patient.
• TheZenithTX2DissectionEndovascularGraftwithPro-FormandtheZ-Trak
Plus Introduction System should only be used by physicians and teams
trained in vascular interventional techniques (catheter-based and surgical)
and in the use of this device. Specific training expectations are described in
Section 10.1, Physician Training.
• Thelong-termperformanceofendovasculargraftshasnotyetbeen
established. All patients should be advised that endovascular treatment
requires life-long, regular follow-up to assess their health and the
performance of their endovascular graft. Patients with specific clinical
findings (e.g., endoleaks, enlarging aneurysms, persisting flow in false lumen,
or changes in the structure or position of the endovascular graft) should
receive enhanced follow-up. Specific follow-up guidelines are described in
Section 12, IMAGING GUIDELINES AND POSTOPERATIVE FOLLOW-UP.
• Afterendovasculargraftplacement,patientsshouldberegularlymonitored
for perigraft flow or changes in the structure or position of the endovascular
graft. At a minimum, annual imaging is required, including: 1) thoracic
device radiographs to examine device integrity (separation between
components or stent fracture); and 2) contrast and non-contrast CT to
examine perigraft flow, patency, tortuosity, device position and progressive
disease. If renal complications or other factors preclude the use of image
contrast media, use of other imaging modalities (e.g., TEE, IVUS) should be
considered in combination with non-contrast CT.
• TheZenithTX2DissectionEndovascularGraftwithPro-Formandthe
Z-Trak Plus Introduction System is not recommended in patients unable to
undergo, or who will not be compliant with, the necessary preoperative and
postoperative imaging and implantation studies as described in Section 12,
IMAGING GUIDELINES AND POSTOPERATIVE FOLLOW-UP.
• Additionalendovascularinterventionsorconversiontostandardopen
surgical repair following initial endovascular repair should be considered for
patients experiencing unacceptable decrease in fixation length (vessel and
component overlap) and/or endoleak.
• Patientsexperiencingreducedbloodflowthroughthegraftand/orleaks
may be required to undergo secondary endovascular interventions or
surgical procedures.
• Alwayshaveaqualifiedsurgeryteamavailableduringimplantationor
reintervention procedures in the event that conversion to open surgical
repair is necessary.
• Interventionssuchasdefibrillation,cardioversion,orCPR,althoughnot
specifically evaluated in studies, may have the potential to disrupt position
or seal of the endograft, and should be followed by imaging to confirm
continued device function.
4.2 Patient Selection, Treatment and Follow-Up
• Accessvesseldiameter(measuredinnerwalltoinnerwall)andmorphology
(tortuosity, occlusive disease, and/or calcification) should be compatible
with vascular access techniques and introduction systems of the profile
of a 20 French or 22 French vascular introducer sheath. Vessels that are
significantly calcified, occlusive, tortuous or thrombus-lined may preclude
femoral introduction of the endovascular graft and/or may increase the risk
of embolization.
• Keyanatomicelementsthatmayaffectsuccessfulexclusionofthedissection
include severe angulation (localized angulation > 45 degrees); short
proximal fixation site (< 20 mm); an inverted funnel shape at the proximal
fixation site (greater than 10% increase in diameter over 20 mm of fixation
site length); and circumferential thrombus and/or calcification at the arterial
fixation sites. Irregular calcification and/or plaque may compromise the
attachment and sealing at the fixation site. Necks exhibiting these key
anatomic elements may be more conducive to graft migration.
• TheZenithTX2DissectionEndovascularGraftwithPro-FormandtheZ-Trak
Plus Introduction System is not recommended for patients who cannot
tolerate contrast agents necessary for intraoperative and postoperative
follow-up imaging.
• TheZenithTX2DissectionEndovascularGraftwithPro-FormandtheZ-Trak
Plus Introduction System is not recommended for patients whose weight or
size would compromise or prevent the necessary imaging requirements.
• Graftimplantationmayincreasetheriskofparaplegiawheregraftexclusion
covers the origins of dominant spinal cord or intercostal arteries.
• Patientswithconnectivetissuedisordershavenotbeenevaluated.
• Highly patent intercostal aortic branches or large collateral vessels are
likely to result in retrograde flow after thoracic graft implantation. Patients
with uncorrectable coagulopathy may also have an increased risk of Type II
endoleak or bleeding complications.
4.3 Implant Procedure
• Systemicanticoagulationshouldbeusedduringtheimplantation
procedure based on hospital and physician preferred protocol. If heparin is
contraindicated, an alternative anticoagulant should be used.
• Minimizehandlingoftheconstrainedendoprosthesisduringpreparation
and insertion to decrease the risk of endoprosthesis contamination and
infection.
• Toactivatethehydrophiliccoatingontheoutsideofthesheath,thesurface
must be wiped with sterile gauze pads soaked in saline solution. Always
keep the sheath hydrated for optimal performance.
• Maintainwireguidepositionduringintroduction system insertion.
• Donotbendorkinktheintroduction system. Doing so may cause damage
to the introduction system and the Zenith TX2 Dissection Endovascular Graft
with Pro-Form.
• Alwaysusefluoroscopyforguidance,delivery,andobservationofthe
Zenith TX2 Dissection Endovascular Graft with Pro-Form and the Z-Trak Plus
Introduction System within the vasculature.
• TheuseoftheZenithTX2DissectionEndovascularGraftwithPro-Formand
the Z-Trak Plus Introduction System requires administration of intravascular
contrast. Patients with pre-existing renal insufficiency may have an increased
risk of renal failure postoperatively. Care should be taken to limit the amount
of contrast media used during the procedure.
• Toavoidtwistingtheendovasculargraft,neverrotatetheintroduction
system during the procedure. Allow the device to conform naturally to the
curves and tortuosity of the vessels.
• Asthesheathiswithdrawn,anatomyandgraftpositionmaychange.
Constantly monitor graft position and perform angiography to check
position as necessary.
• Inaccurateplacementand/orincompletesealingoftheZenith TX2
Dissection Endovascular Graft with Pro-Form within the vessel may result
in increased risk of endoleak, migration, or inadvertent occlusion of the left
subclavian, left common carotid, and/or celiac arteries.
• Incorrectdeploymentormigrationoftheendoprosthesismayrequire
surgical intervention.
• Do not continue advancing the wire guide or any portion of the introduction
system if resistance is felt. Stop and assess the cause of resistance; vessel,
catheter, or graft damage may occur. Exercise particular care in areas of
stenosis, intravascular thrombosis, or calcified or tortuous vessels.
• Unlessmedicallyindicated,donotdeploytheZenith TX2 Dissection
Endovascular Graft with Pro-Form in a location that will occlude arteries
necessary to supply blood flow to organs or extremities. Do not cover
significant arch or mesenteric arteries (exception may be the left subclavian
artery) with the endoprosthesis. Vessel occlusion may occur. If a left
subclavian artery is to be covered with the device, the clinician should
be aware of the possibility of compromise to cerebral and upper limb
circulation.
• Usecautionduringmanipulationofcatheters,wiresandsheathswithina
dissection. Significant disturbances may dislodge fragments of thrombus,
which can cause distal or cerebral embolization.
• Avoiddamagingthegraftordisturbinggraftpositioningafterplacement
in the event reinstrumentation (secondary intervention) of the graft is
necessary.
• Donotattempttore-sheaththegraftafterpartialorcompletedeployment.
• Repositioningthestentgraftdistallyafterpartialdeploymentofthecovered
proximal stent may result in damage to the stent graft and/or vessel injury.
• Toavoidimpalinganycathetersleftinsitu,rotatetheintroduction system
during withdrawal.
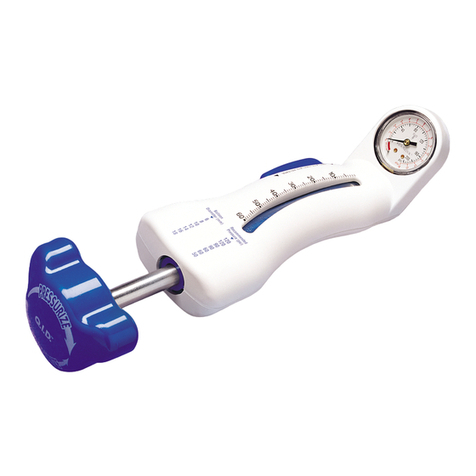
4.4 Molding Balloon Use - Optional
• Confirmcompletedeflationofballoonpriortorepositioning.
• Do not inflate balloon in aorta outside of graft.
• Usecautionduringmoldingwithinadissection
• Foraddedhemostasis,theCaptor™HemostaticValvecanbeloosenedor
tightened to accomodate the insertion and subsequent withdrawal of a
molding balloon.