18
ENGLISH
s
Note
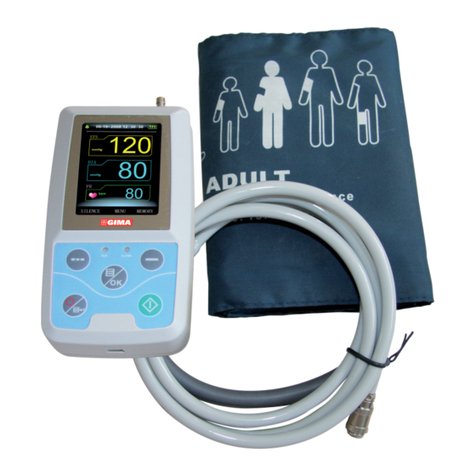
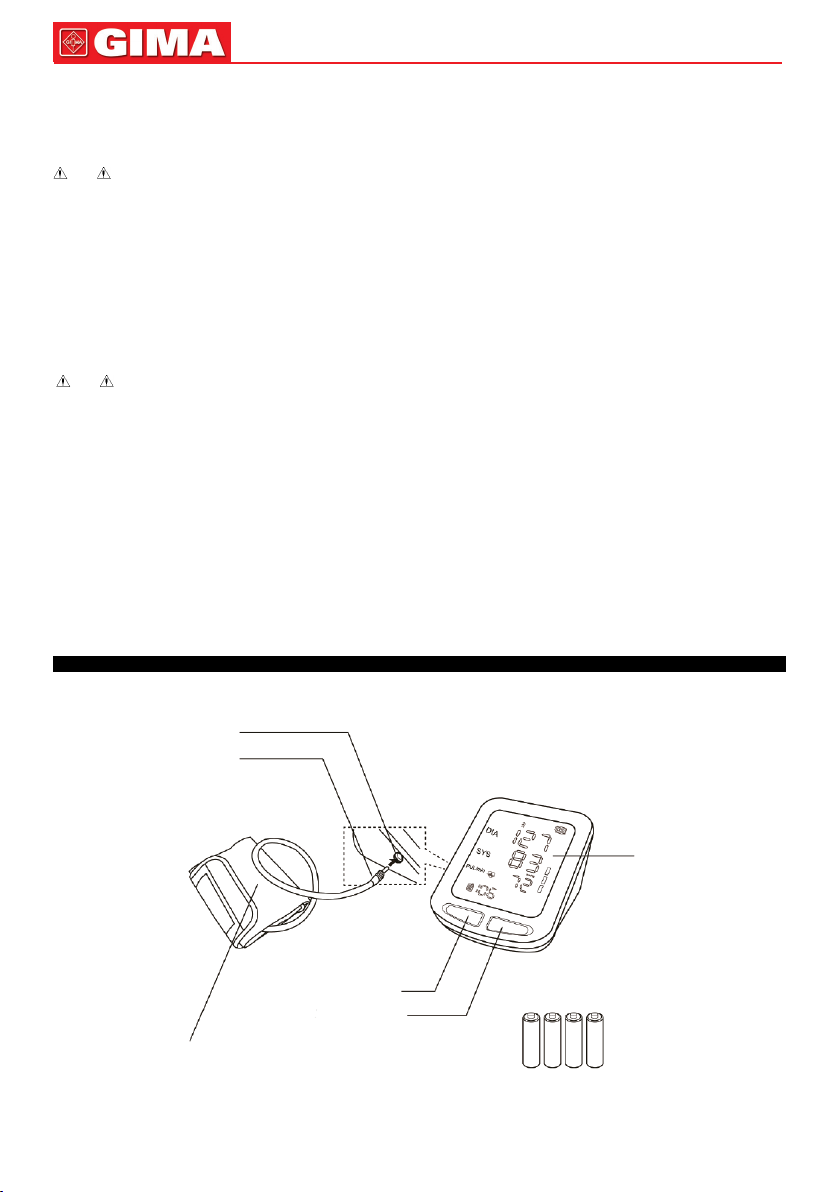
All the operaons to the Electronic Sphygmomanometer are through buons. The names of the buons are above them. They are:
Le buon is “M” buon, under “OFF” state, press this buon to enter the review interface (refer to Chapter 8 for details.).
Right buon is “START/STOP” buon, under “OFF” state, press this buon to enter measurement mode, inate the cu to measure blood
pressure, press this buon again to turn o the device.
Under “OFF” state, press “M” buon and “START/STOP” buon simultaneously for 5 s to enter the seng interface, the default unit in this interface is
“mmHg”; short press “M” buon to switch the unit between “mmHg” and “kPa”.
Press “START/STOP” buon again in the unit seng interface to enter the volume seng interface. Press “M” buon to change the volume, the
maximum volume is 4, and the minimum is 0 (silence).
Aer compleng the seng, repeatedly press the “START/STOP” buon to turn the device o.
Note
The default unit of the device when leaving factory is mmHg.
In the volume seng interface, press “START/STOP” buon to enter the factory seng interface, in which the “CAL” is the stac pressure interface,
and the “FAC” is the aging interface, which does not require user to operate. If you want to end the interface, press the “START/STOP” buon
twice to turn the device o.
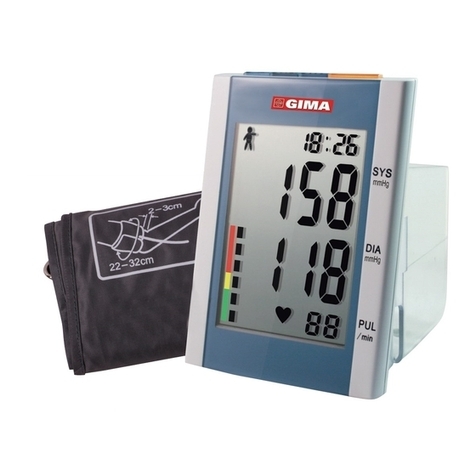
1. Adopt a comfortable sing posion, use back and arms to support the body.
2. Place your elbow on a table, the palm faces up and the body is relaxed.
3. The cu is level with your heart.
4. Feet at on the oor, and do not cross your legs.
The high and low locaon of cu will cause changes in measure results.
Do not touch the sphygmomanometer, cu and windpipe during measure.
Remain sll 4~5 minutes before measurement.
Do not talk and movement during the measurement. Relax the body, do not let the muscle acvity.
Wait 4~5 minutes between measurements.
Do not use precision instrument near the Sphygmomanometer.
When repeatedly measuring, the accurate blood pressure value may not be measured due to congeson in the arm. Please measure aer the blood
ow is smooth.
Repeated measurement for a long period of me, limbs rubbing with the cu may be accompanied by purpura, ischemia and nerve damage. When
measurement a paent, it is necessary to frequently check the color, warmth and sensivity of the distal of the limb. Once any abnormalies are
observed, place the cu in another posion or stop the blood pressure measurement immediately.
Please use the device at an environment of suitable temperature and humidity otherwise it will cause measurement error.
Do not twist or wrap the airway tube. It can cause constant pressure in the cu which can block blood ow and cause serious damage to the paent.
Do not use the cu on the injured area, which will cause more serious damage to the area.
Do not use the cu in the area where the treatment is being performed inside blood vessel or the arteriovenous connecon. This may cause
temporary blockage of blood ow and cause injury to the paent.
Do not use the cu on the side of the mastectomy.
When using the cu to pressurize, some of the body’s funcons may temporarily weaken. Do not use the measurement medical electrical equipment
at the appropriate arm posion.
Do not move during measurement, it will have a delayed eect on the paent’s blood ow.
The device need to be placed for 2 hours from the minimum storage temperature to being ready for its intended use.
The device need to be placed for 4 hours from the highest storage temperature to being ready for its intended use.
Note
Take the measurement in one hour aer meal or aer drinking alcohol, coee or aer smoking, exercise, bathing;
Using incorrect posture such as standing or lying down, etc;
The paent speak or move his body during measurement;
When measuring, the paent is nervous, excited, emoonal instability;