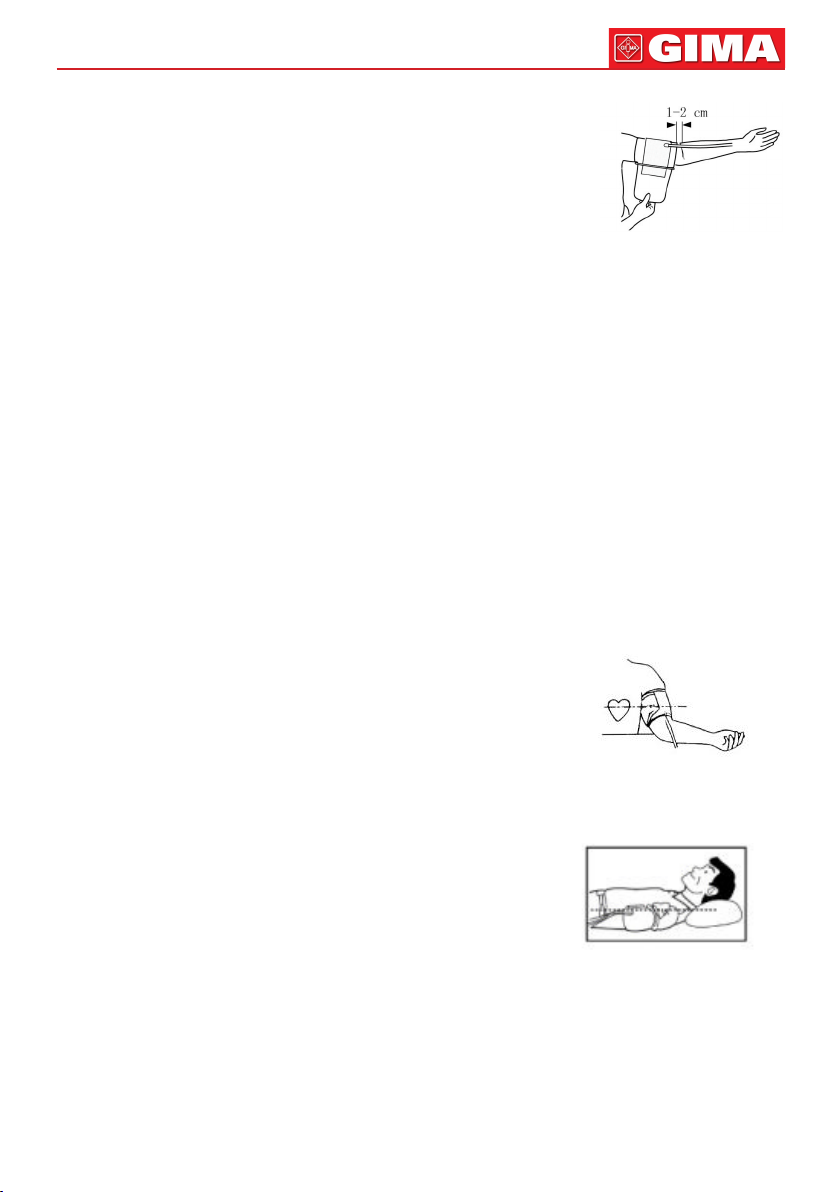
26
to allow the blood circulation in your arm to recover. Prolonged
over-ination (cuff pressure exceed 300 mmHg or maintained above15
mmHg for longer than 3 minutes) of the bladder may cause ecchymoma
of your arm.
7. Consult your physician if you have any doubt about below cases:
1 The application of the cuff over a wound or inammation diseases;
2 The application of the cuff on any limb where intravascular access
or therapy, or an arterio-venous (A-V) shunt, is present;
3 The application of the cuff on the arm on the side of a mastectomy;
4 Simultaneously used with other monitoring medical equipments
on the same limb;
5 Need to check the blood circulation of the user.
8. This Electronic Sphygmomanometers is designed for adults and
should never be used on infants or young children. Consult your physician
or other health care professionals before use on older children.
9. Do not use this unit in a moving vehicle, This may result in erroneous
measurement.
10.Blood pressure measurements determined by this monitor are equivalent
to those obtained by a trained observer using the cuff/stethoscope
auscultation method, within the limits prescribed by the American
National Standard Institute, Electronic or automated sphygmomanometers.
11.Information regarding potential electromagnetic or other interference
between the blood pressure monitor and other devices together
with advice regarding avoidance of such interference please see part
ELECTROMAGNETIC COMPATIBILITY INFORMATION.
12.If Irregular Heartbeat (IHB) brought by common arrhythmias is detected
in the procedure of blood pressure measurement, a signal of ‘(♥)’will be
displayed. Under this condition, the Electronic Sphygmomanometers
can keep function, but the results may not be accurate, it’s suggested
that you consult with your physician for accurate assessment.
There are 2 conditions under which the signal of IHB will be displayed:
1 The coefcient of variation (CV) of pulse period >25%.
2 The difference of adjacent pulse period≥0.14s, and the number of such
pulse takes more than 53 percentage of the total number of pulse.
13.Please do not use the cuff other than supplied by the manufacturer,
otherwise it may bring biocompatible hazard and might result in
measurement error.
14. The monitor might not meet its performance specications or cause
safety hazard if stored or used outside the specied temperature and
humidity ranges in specications.
15. Please do not share the cuff with other infective person to avoid
cross-infection.
ENGLISH