36
measured data is beyond the highest or lowest limit, the
device would start alarming automatically on the premise
of the alarming function is on.
The device has the function of alarming, this function can
either be paused, or closed (default setting) for good.
This function could be turned on through menu operation
if you need. Please check the chapter 6.2 as a reference.
The device may not work for all patients. If you are una-
ble to achieve stable readings, discontinue use.
A exible circuit connects the two parts of the device. Do
not twist or pull on the connection.
2 OVERVIEW
The pulse oxygen saturation is the percentage of HbO2in
the total Hb in the blood, so-called the O2concentration in
the blood. It is an important bio-parameter for the respira-
tion. A number of diseases relating to respiratory system
may cause the decrease of SpO2in the blood, furthermore,
some other causes such as the malfunction of human body’s
self-adjustment, damages during surgery, and the injuries
caused by some medical checkup would also lead to the
difculty of oxygen supply in human body, and the corre-
sponding symptoms would appear as a consequence, such
as vertigo, impotence, vomit etc. Serious symptoms might
bring danger to human’s life. Therefore, prompt information
of patients’ SpO2is of great help for the doctor to discover
the potential danger, and is of great importance in the clinical
medical eld.
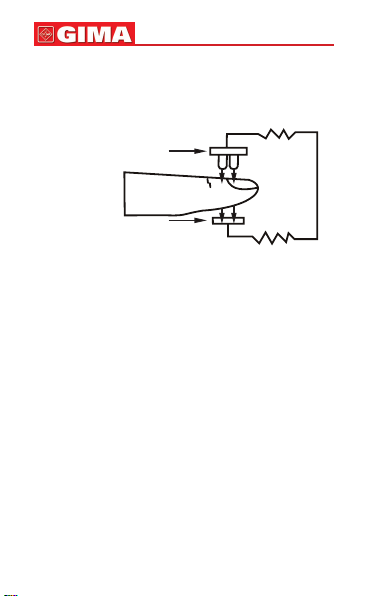
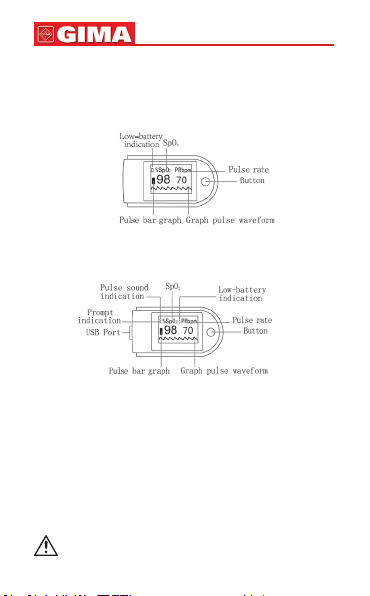
The Pulse Oximeter features in small volume, low power
consumption, convenient operation and being portable. It is
only necessary for patients to put one of his ngers into a
probe for diagnosis, and a display screen will directly show
the measured value of pulse oxygen saturation with the high
veracity and repetition.
2.1 Classication:
Class II b(MDD93/42/EEC IX Rule 10)
ENGLISH