15 ENGLISH
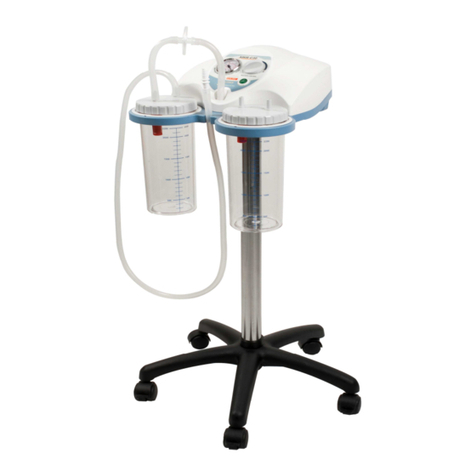
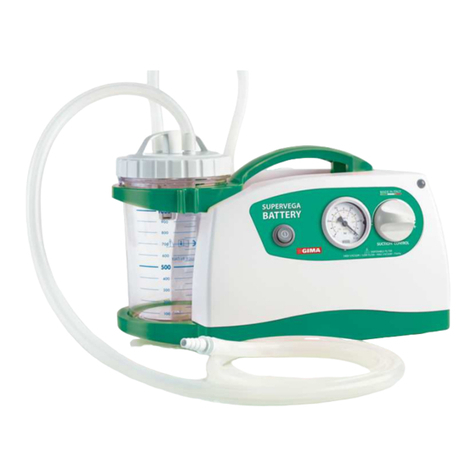
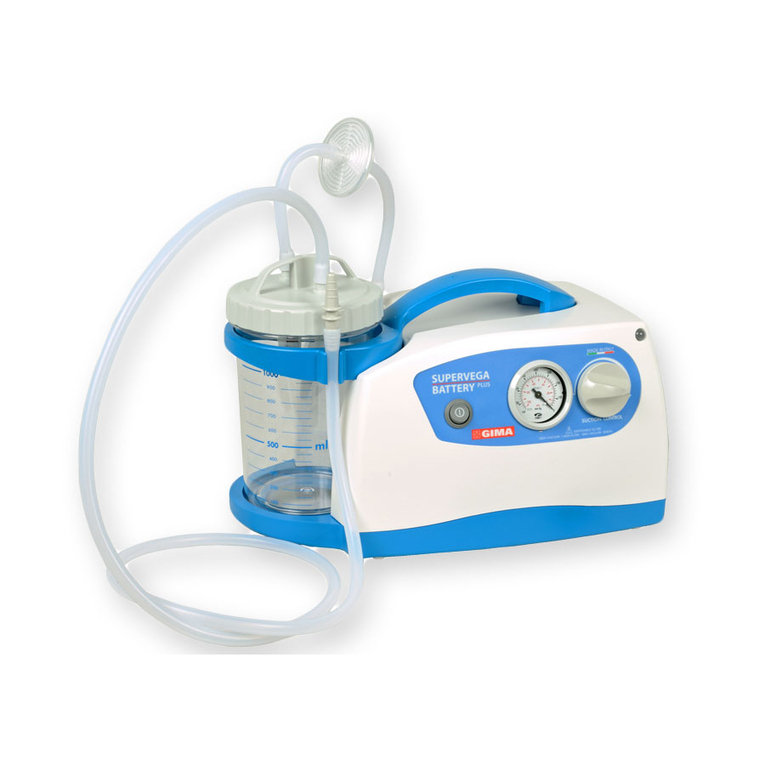
SUPERVEGA BATTERY SUCTION UNIT is a surgical suction pump with the following electrical character-
istics: 14V
4A with AC/DC (input: 100-240V~ - 50-60Hz – 1.5A) to be used for nasal, oral and tracheal
aspirations in adults or for body liquids in children (for example mucus, phlegm and blood) equipped with 5
wheels with braking device. Thanks to these characteristics and its high performance levels, this device is
particularly suitable for use in hospital wards, in small surgical applications and post-operative treatments. A
device designed to offer ease-of-transport and almost continuous use thanks to the adoption of an electronic
system to manage the power supply. Supplied with an audible alarm and visual tell-tale (luminous LED) to
indicate the battery status. Featuring a body in plastic with high thermal and electrical insulation in compliance
with recently introduced European safety regulations. Supplied with no. 2 complete sterilisable polycarbonate
jugs with overow valve. Features a suction regulator and vacuum gauge located on the front panel.
GENERAL WARNING
Read instruction manual carefully before use.
Only highly qualied staff use reserved.
The instrument must not be disassembled. For a technical service always contact Gima.
Keep off the reach of children or not capable people without supervision.
Full containers must be handled with great care during transfer to the disposal areas, following the local
procedures and regulations.
IMPORTANT SAFETY RULES
1. Check the condition of the unit before each use. The surface of the unit should carefully be inspected for
visual damage. Check the mains cable and do not connect to power if damage is apparent;
2. Before connecting the appliance always check that the electric data indicated on the data label and the type
of plug used, correspond to those of the mains electricity to which it’s to be connected;
3. Respect the safety regulations indicated for electrical appliances and particularly:
- Use original components and accessories provided by the manufacturer to guarantee the highest ef-
ciency and safety of the device;
- The device can be used only with the bacteriological lter;
- Never immerge the appliance into water;
- Position the device on stable and at surfaces in a way that the air inlets on the back aren’t obstructed;
- To avoid incidents, do not place the aspirator on unstable surfaces, which may cause it to accidentally fall
and lead to a malfunction and/or breakage. Should there be signs of damage to the plastic parts, which
may expose inner parts of the energised device, do not connect the plug to the electrical socket. Do not
attempt to make the device work before it has been thoroughly checked by qualied personnel and/or the
GIMA technical service department.
- Don’t use in the presence of inammable substances such as anaesthetic, oxygen or nitrous oxide;
- Don’t touch the device with wet hands and always prevent the appliance coming into contact with liquids;
- Don’t leave the appliance connected to the power supply socket when not in use;
- Don’t pull the power supply cable to disconnect the plug remove the plug from the mains socket correctly;
- Store and use the device in places protected against the weather and far from any sources of heat. After
each use, it is recommended to store the device in its own box away from dust and sunlight.
- In general, it is inadvisable to use single or multiple adapters and/or extensions. Should their use be
necessary, you must use ones that are in compliance with safety regulations, however, taking care not to
exceed the maximum power supply tolerated, which is indicated on the adapters and extensions.
- Prevent children from using the device without proper supervision;
4. For repairs, exclusively contact technical service and request the use of original spare parts. Failure to
comply with the above can jeopardise the safety of the device;
5. Use only for the purpose intended. Don’t use for anything other than the use dened by the manufacturer.
The manufacturer will not be responsible for damage due to improper use or connection to an electrical
system not complying with current regulation.
6. The medical device requires special precautions regarding electromagnetic compatibility and must be in-
stalled and used in accordance with the information provided with the accompanying documents: the SU-
PERVEGA BATTERY SUCTION UNIT device must be installed and used away from mobile and portable
RF communication devices (mobile phones, transceivers, etc.) that may interference with the said device.
7. WARNING: Do not change this equipment without the permission of the manufacturer GIMA S.p.A. None
of electric or mechanical parts has been designed to be repaired by customers or end-users. Don’t open