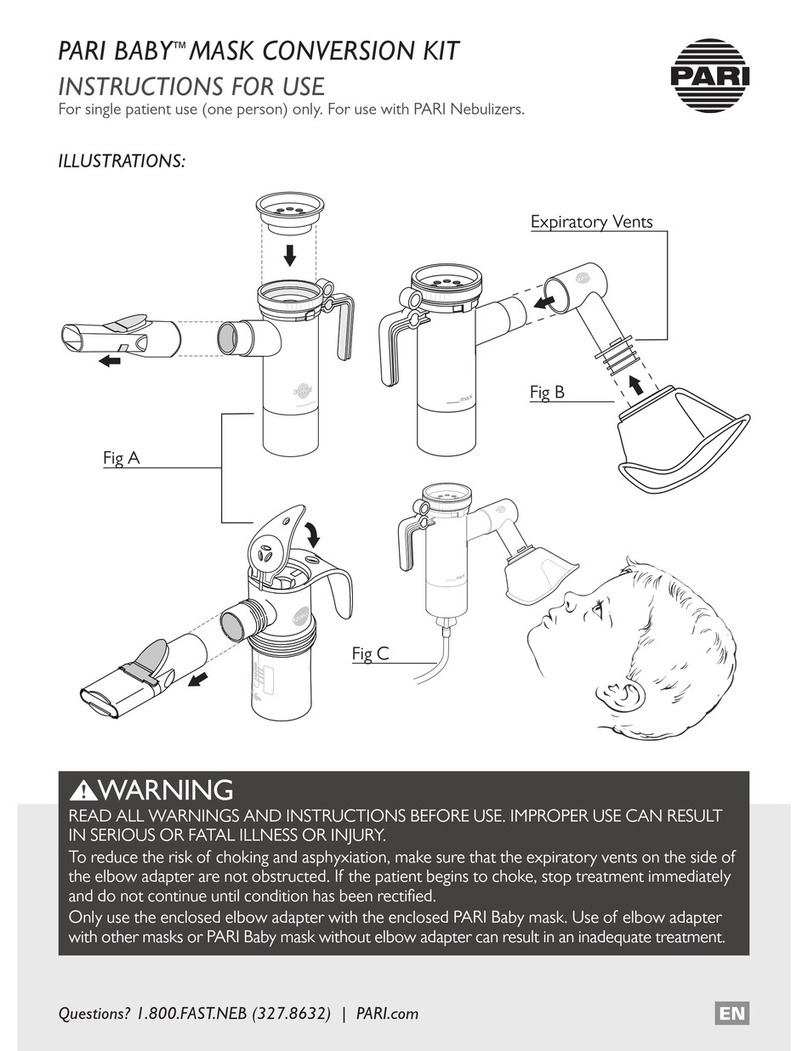
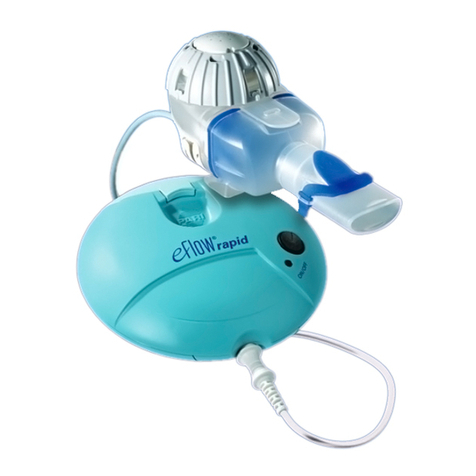
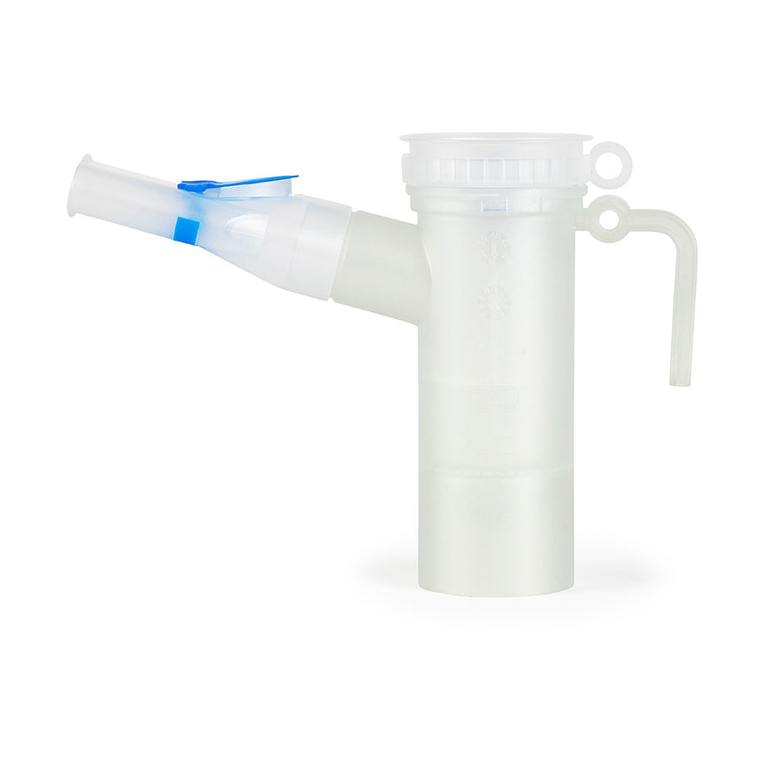
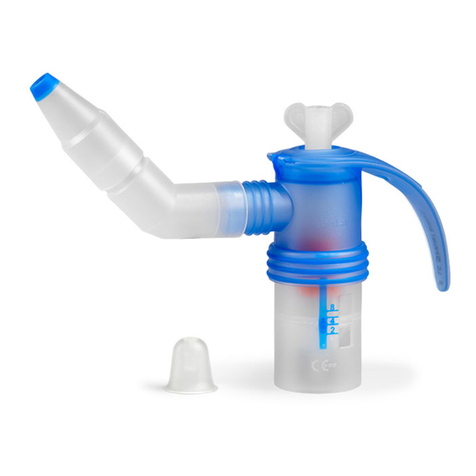
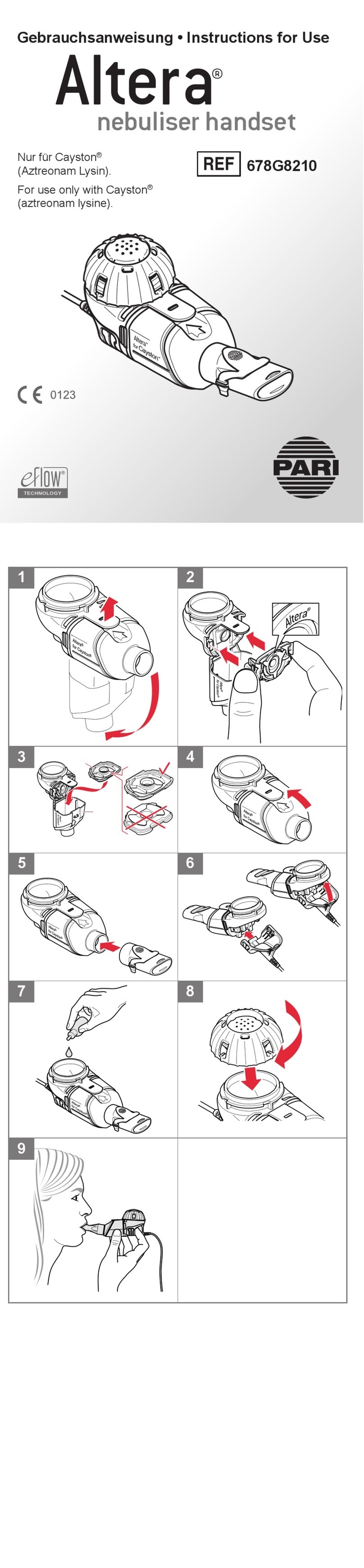
eFlow®rapid - 2021-04 5
en
1IMPORTANT INFORMATION
Read these instructions for use and the
instructions for use of the accompanying
accessories completely before using for
the first time. Keep them safe so that you
can refer to them again later.
Report serious incidents to the manufac-
turer and the competent authorities.
You should have a medical examination
before treating illnesses.
Organisation of warnings
In these instructions for use, safety-
related warnings are organised into
hazard levels:
- The signal word WARNING is used to
indicate dangers that may result in seri-
ous injuries or even death if precaution-
ary measures are not taken.
- The signal word CAUTION is used to
indicate dangers that may result in slight
to moderate injuries or impair treatment
if precautionary measures are not
taken.
- The signal word NOTE is used to indi-
cate general precautionary measures
for working with the product that should
be followed to avoid damaging the
product.
Handling the nebuliser
system
Check the nebuliser system before every
use.
Follow the instructions below in order to
operate the nebuliser system safely:
- Always remove the power adapter from
the socket to ensure that the power is
completely cut off.
- Keep the nebuliser system away from
hot surfaces (e.g., hob).
- Do not allow pets near the cables.
- Do not use the nebuliser system in
areas where there is a risk of explosion
or in the presence of gases that promote
combustion (e.g., oxygen, nitrous oxide,
flammable anaesthetics).
- Do not carry out an inhalation session
while you are driving a motor vehicle
(risk of accident).
CAUTION:
If these instructions for use are not fol-
lowed, injuries and damage to the product
cannot be ruled out.
CAUTION:
Replace all broken, deformed and heavily
discoloured parts. Damaged parts can
impair the function of the nebuliser system
and thus also affect treatment.
WARNING:
Do not operate the nebuliser system if the
power adapter is visibly damaged, since
otherwise there is a danger from contact
with live parts (e.g., electric shock).
WARNING:
Never remove the power adapter from
the socket with wet hands. There may
be a danger of electric shock.