Introduction
Dear user You are kindly invited to read this manual carefully before proceeding to use the Product in order to
safeguard yourself and other people from any injuries.
This appliance is a Class 1 medical device pursuant to European Directives on medical devices (MDD) 93/42/EEC,
Annex IX, and 2007/47/EC.
The manufacturer declares that this product is in compliance with Annex I (essential requirements of Directive
93/42/EEC and certifies such conformity by affixing the CE mark.
This operator’s manual refers to the product GIMANORD.
The customer service is at your disposal in case of Product details, information concerning its use, identification of
spare parts being required and for any other queries you might have concerning the appliance, for ordering spares
and for matters relating to assistance and warranty.
GIMA TECHNICAL ASSISTANCE OFFICE FOR CLIENTS
Via Marconi, 1 –20060 GESSATE (MI) ITALY
Tel. +39 02 953854209 Fax +39 02 95381167
The contents of this Manual may be amended by GIMA, without prior notice or any further obligations, in order to
make changes and improvements. The reproduction, including partial, or translation of any part of this manual is
forbidden without the written permission of GIMA.
GIMA reserves the right to change, cancel or otherwise amend the data contained in this document at any time and
for any reason without prior notice inasmuch as GIMA is constantly seeking new solutions which lead to product
evolution. GIMA therefore reserves the right to make changes to the supplied Product in terms of shape, fittings,
technology and performances.
With regard to translations into languages other than Italian, reference shall always be made to the Italian edition of
this operator’s manual.
1. General information
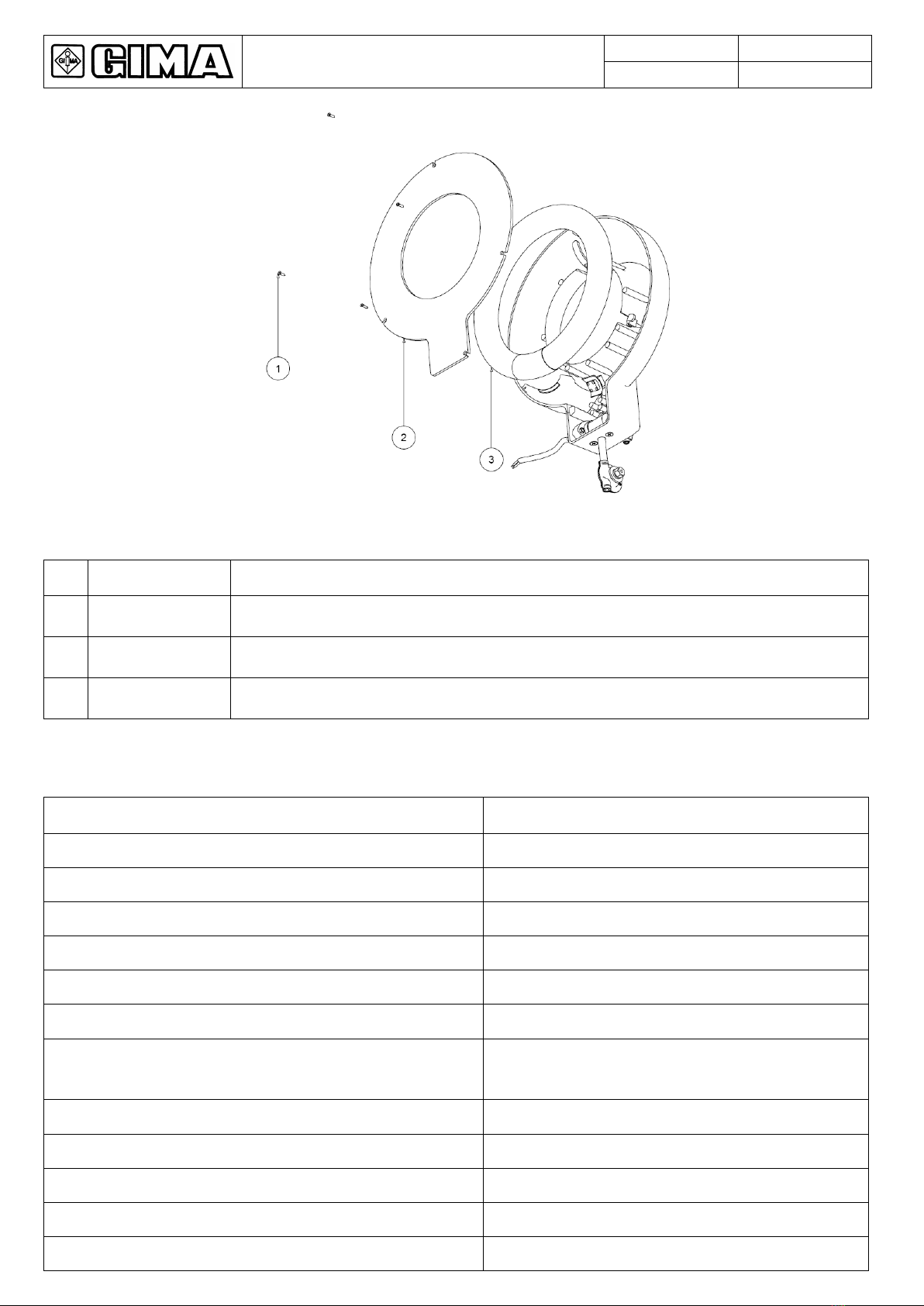
The ME (Medical-Electrical) EQUIPMENT to which this manual refers is a LAMP for diagnosis or observation.
For easier description such ME EQUIPMENT will be indicated in this manual with the name of “Product”.
This manual is an integral part of the Product as required by European directive 93/42/EEC and 2007/47/EC.
Always keep this installation manual close to the Product.
The Product is not suitable for use in explosion-risk areas
- The Product is not suitable for use in the presence of inflammable mixtures of anaesthetics with air, oxygen or
NO2(laughing gas)
GIMA disclaims all liability for any injuries to persons or damage to things caused by the installation,
maintenance or use of the Product by unqualified operators. By qualified operator is meant whosoever has attended
a course relating to the installation, maintenance and use of the product organised by GIMA or, alternatively,
whosoever has carefully read this installation manual.
The only party responsible for Product installation is the buyer’s customer itself; no cost or responsibility relating to
installation and/or commissioning of the Product shall therefore be traced back and/or in any case attributed to
GIMA.
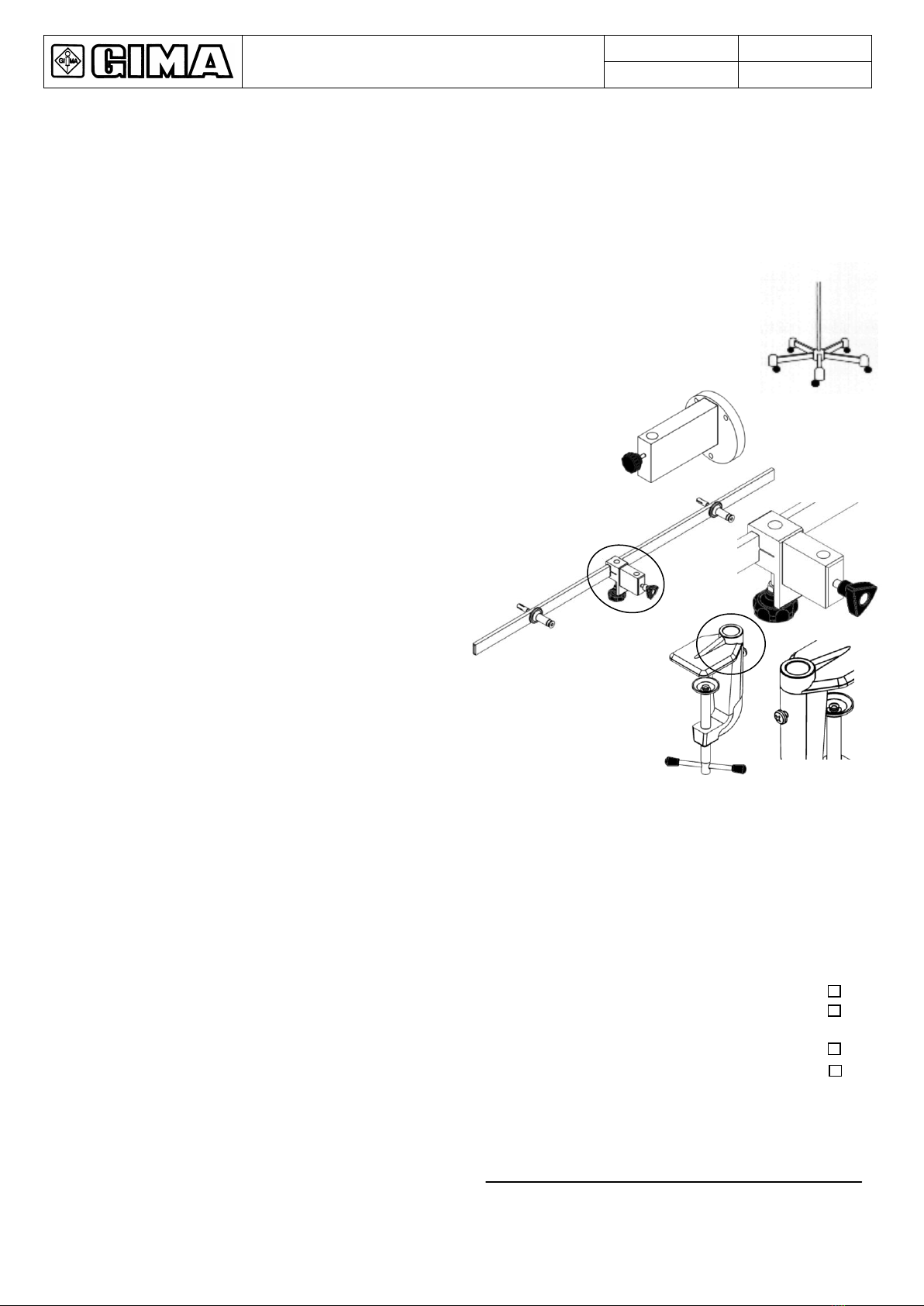
The masonry works involving the preparation of the ceiling or wall, for Products to be installed on the ceiling or
wall respectively, and the electric works for preparing the power supply system for the Product shall be of a sturdy
and safe nature and completed in a workmanlike manner by suitably trained personnel.
By way of example only, without limitation, the following professional figures are deemed adequately trained:
Building Engineer, Draughtsman, Building firm duly registered in the professional Register (for masonry works)
Electro-technician qualified to exercise the profession of electrician (for the electrical works)
The Product is a ME EQUIPMENT and consequently falls within the field of application of the EN:62353 standard.
Consequently, any operation performed on the Product must be carried out in compliance with the EN:62353
standard, where applicable.