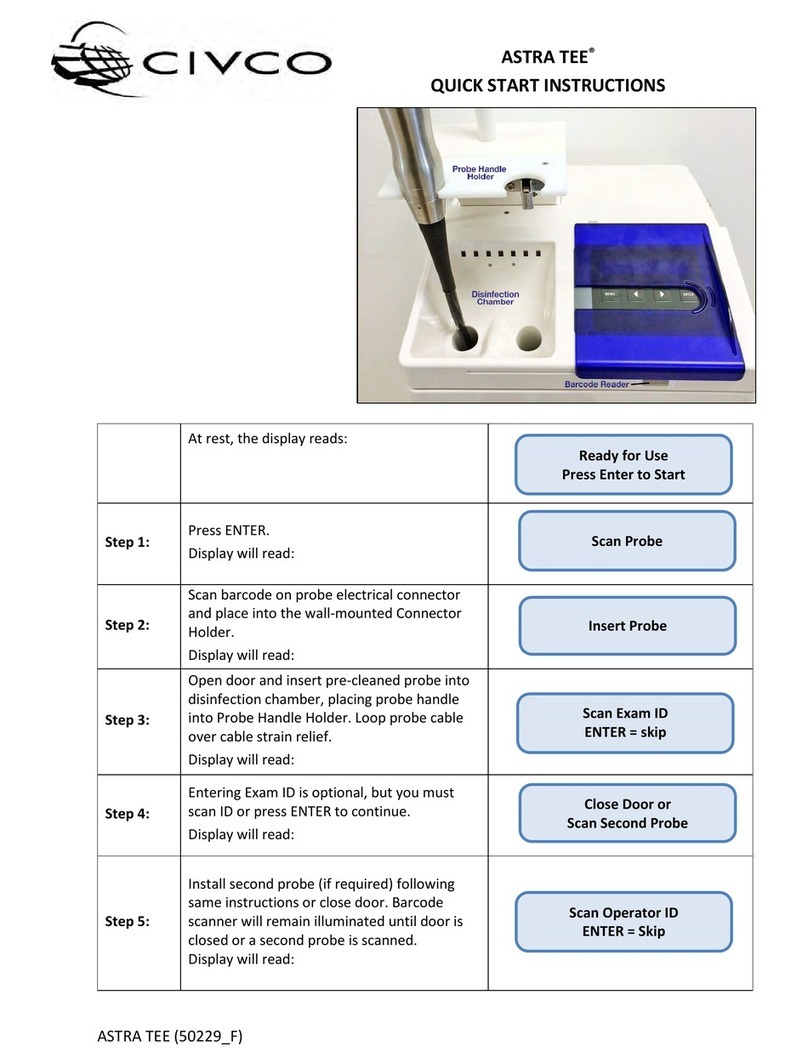
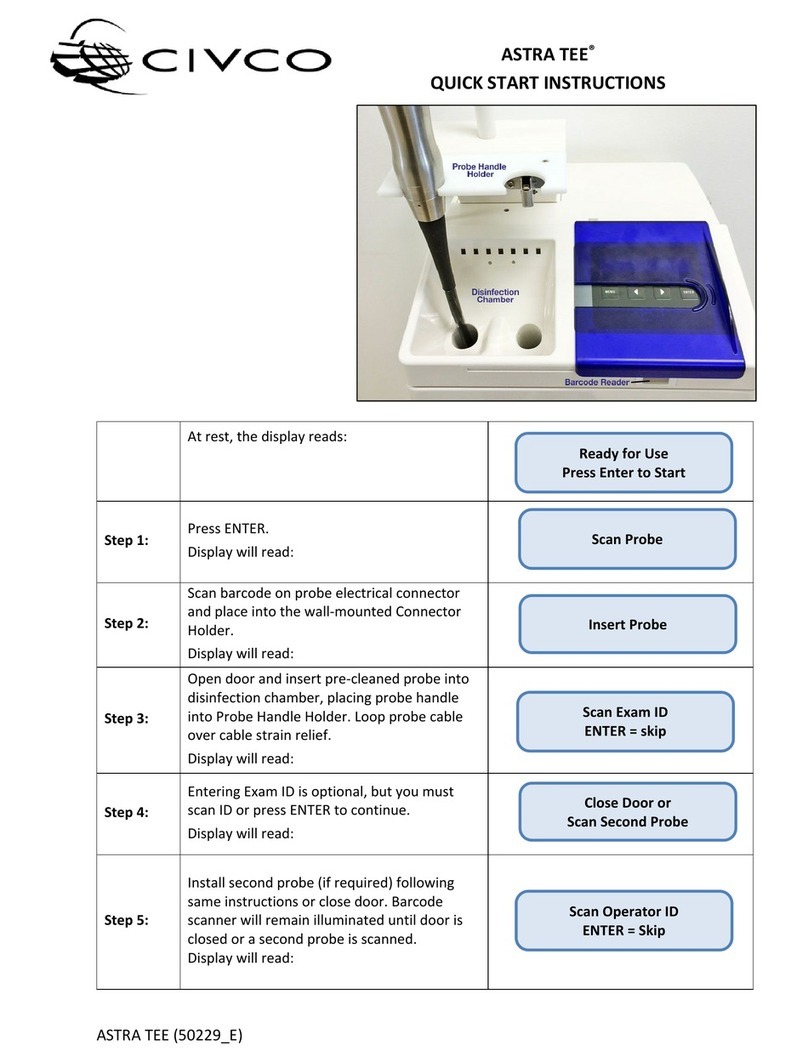
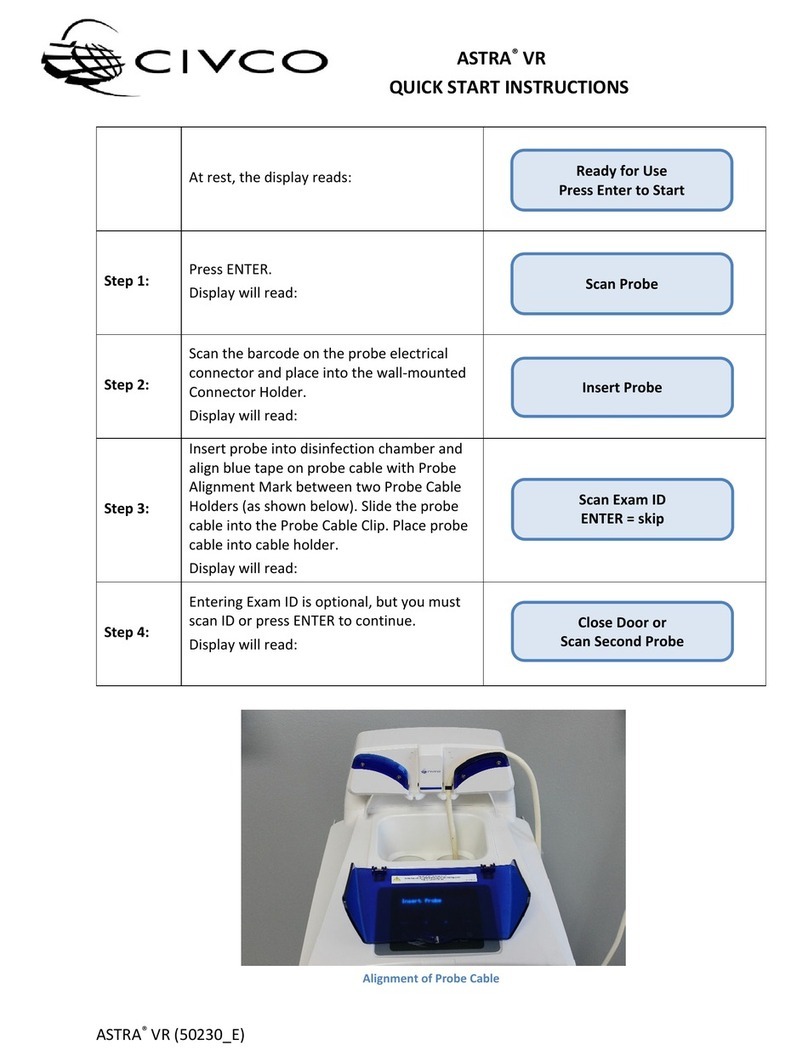
ASTRA
ULTRA
ASTRA
PLUS
ASTRA
BASE
Sourcing of HLD and Test Strips CIVCO Any
Vendor
Any
Vendor
Additional HLD and Test Strip Tracking Features
Logs Lot # and Manufacturer's Expiration Date √ √ -
Prevents Use Beyond Manufacturer's Expiration Date √ √ -
Confirms Test Strip Compatible with HLDSolution √ √ -
Provides MRC Test Countdown Timer on screen display during daily cycles √ √ -
Mandates Quality Control for Newly Opened Test Strip Bottles and Logs Results √- -
Logs Date Test Strip Bottles Opened √- -
Prevents Use Beyond Test Strip Open Shelf-Life √- -
C. WARNINGS & CAUTIONS
CAUTION:
lRead the entire manual before using the device.
lThere are no user serviceable parts inside. Shock hazard may be present behind access panel. Panel removal
should be performed only by qualified service personnel.
lDo not use this system until it has been properly installed.
lUse the device only for the intended use as described in this manual.
lDo not operate the device if it is not working properly or if any part of the device has been dropped or
damaged.
lFor use only with CIVCO Medical Solutions approved high-level disinfectants.
lConnect the system to a hospital grade GFCI receptacle only. Confirm GFCI outlet is properly grounded prior to
use. The GFCI outlet must be tested on a regular basis per the GFCI manufacturer’s recommendation.
lDo not attempt to neutralize the high-level disinfectant while inside the disinfection chamber.
lDo not operate the system if fluid leakage is present - contact CIVCO Medical Solutions.
lThis device is not intended to augment or replace proper pre-cleaning prior to disinfection. If you are unclear
as to the proper pre-cleaning method for your probe, please contact the probe manufacturer for their
recommended procedure.
lFailure to check with the probe manufacturer to determine soaking depth can result in a damaged probe.
lA Water Inlet Line Disinfection (WILD) cycle must be conducted after installing a new water filter. Refer to
Section 3.
lThe high-level disinfectant must be replaced if the minimum required concentration (MRC) test fails or the
reuse life is reached in accordance with the HLD manufacturer’s instructions for use (IFU). Failure to do so can
result in improperly disinfected probes.
lDo not use abrasive cleaning materials, solvents, or alcohol to clean the device.
lAvoid using Sani-Cloth® AF3 wipes to clean unit. It contains an ingredient known to damage the unit.
lDo not pour any items directly into disinfection chamber.
lDo not use this device for any purpose other than its intended use.
lIf you notice any unexplained changes in the performance of the device, or it is making unusual sounds,
contact CIVCO Medical Solutions.
lTip Hazard – Do not use the system without supplied wall bracket.
lDetermine that the subject probe is not damaged and in good working condition in accordance with
manufacturer’s recommendations before using in the ASTRA TEE system.
lWARNING: Portable RF communications equipment (including peripherals such as antenna cables and external
antennas) should be used no closer than 12 inches (30 cm) to any part of the ASTRA unit, including cables
specified by the manufacturer. Otherwise, degradation of the performance of this equipment could result.
lCaution: Exercise care when handling HLD bottle to avoid unnecessary exposure and contact with skin and
eyes. Refer to HLD MSDS for further detail.
M0068_H 3
ASTRA TEE® Operator's Manual