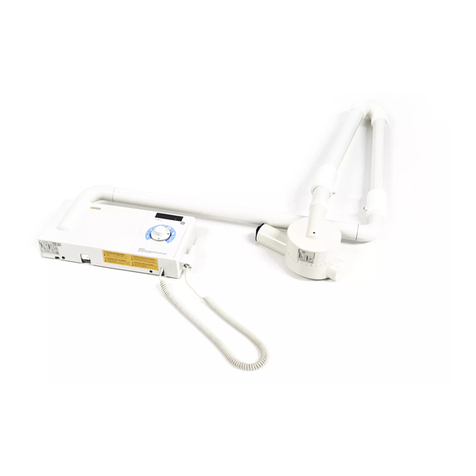
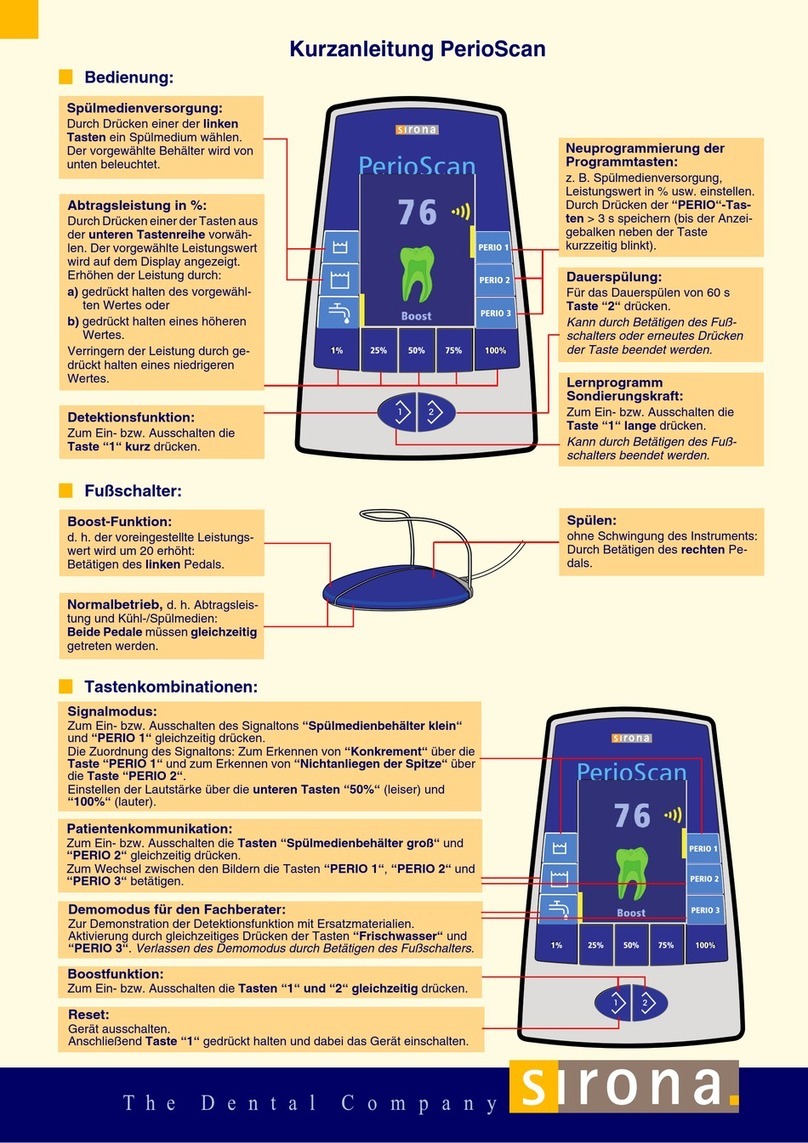
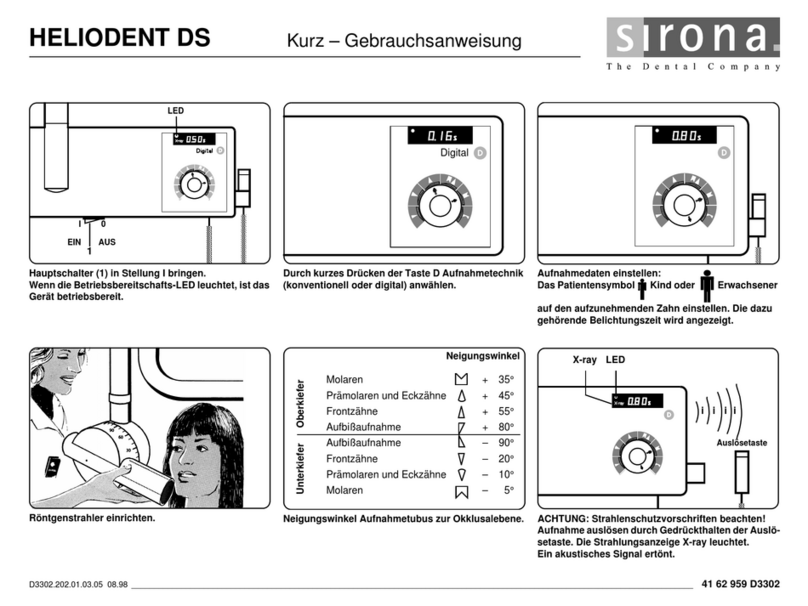
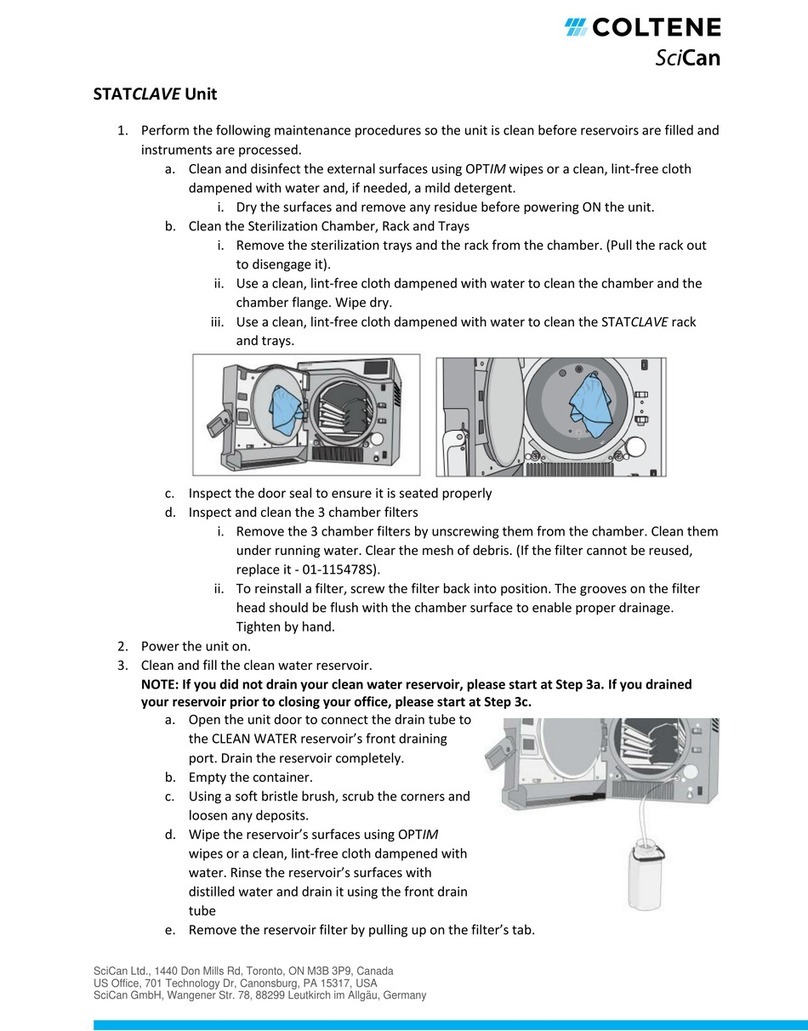
64 57 498 D3543
20 D3543.201.01.05.02 08.2016
2Safety instructions Sirona Dental Systems GmbH
2.17Modifications and extensions of the system Operating Instructions INTEGO
2.17 Modifications and extensions of the system
Modifications to this unit which might affect the safety of the system
owner, patients or other persons are prohibited by law.
For reasons of product safety, this product may be operated only with
original Sirona accessories or third-party accessories expressly approved
by Sirona. The user is responsible for any damage resulting from the use
of non-approved accessories.
If any units not approved by Sirona are connected, they must comply with
the applicable standards, e.g.:
● IEC 60950-1 or IEC 62368-1 for information technology equipment
(e.g., PC, Ethernet switch, Ethernet hub, Ethernet converter, USB
repeater, Ethernet router, USB switch, HDMI repeater/amplifier,
audio sources, display port repeater/amplifier, etc. and their power
supply units), as well as
● IEC 60601-1 for medical devices.
The treatment center monitor must fulfill the requirements of the IEC
60950-1 or IEC 62368-1 standards.
The loudspeaker port of the monitor may only be connected to a device
that complies with IEC 60950-1, IEC 62368-1 (e.g. a PC), or IEC 60601-
1. Under no circumstances may it be connected to a stereo system or
similar.
If a system is created during the installation process, the requirements of
IEC 60601-1, 3 must be fulfilled. The person assembling the system
assumes responsibility for ensuring that it conforms to Directive 93/42/
EEC, for example.
2.18 Electromagnetic compatibility
Medical electrical equipment is subject to special precautionary
measures with regard to electromagnetic compatibility (EMC). They must
be installed and operated as specified in the document “Installation
Requirements”.
Portable and mobile RF communications equipment may interfere with
medical electrical equipment. Therefore, use of such devices (e.g. cellular
phones) in the practice or hospital environments must be prohibited.
HF surgical device with capacitive user interfaces
Operating an HF surgical device
Treatment with HF surgical devices creates strong electromagnetic fields,
which may affect other electronic devices. Do not place external HF
surgical device on the surfaces of the treatment center. Do not run the
cable of the HF handpiece over the surfaces of dentist or assistant
element, user interfaces, or other keys such as the On/Off key on the X-
ray image viewer. Electromagnetic interference can often be reduced by
operating the external HF surgical device with a neutral electrode.
Turn on the treatment center with the Clean button inactive, to avoid
malfunction. See section "Display mode / Clean" [ → 42] on the EasyPad
or "Clean button" [ → 45] on the EasyTouch.