Erbe ICC 350 User manual


ERBOTOM ICC 350 V 4.X
10128-016, 10128-061, 10128-300, 10128-301, 10128-025,10128-402
ERBOTOM ICC 350 Z V 2.X
10128-065
ERBOTOM ICC 350 T V 2.X
10128-066
ERBOTOM ICC 350 M V4.X
10128-080, 10128-083
ERBOTOM ICC 350 M-DOKU V 4.X
10128-081
Instruction manual
08.00

All rights to this instruction manual, particularly the right to reproduction, distribution and translation, are reserved.
No part of this instruction manual may be reproduced in any form (including photocopying, microfilm or other
means), or processed, reproduced or distributed by means of electronic systems without prior written permission
from ERBE ELEKTROMEDIZIN GmbH.
The information contained in this instruction manual may be revised or extended without prior notice and represents
no obligation on the part of ERBE ELEKTROMEDIZIN GmbH.
©ERBE ELEKTROMEDIZIN GmbH, Tübingen 2000
Printed by: ERBE ELEKTROMEDIZIN, Tübingen Instruction manual No. 80104-301
ISO 9001
EN 46001

Chapter Title Page
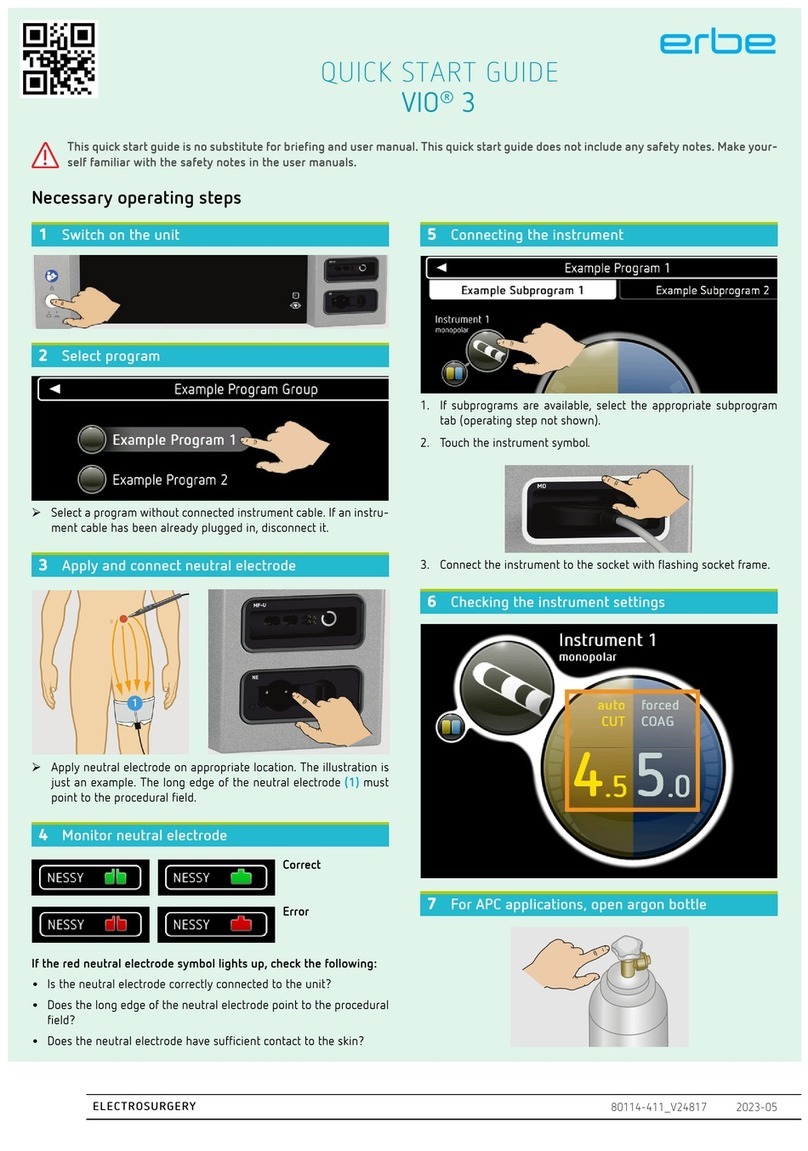
1 INTRODUCTION................................................................ 1-1
1.1 Intended purpose of the ICC 350 ................................................ 1-1
1.2 How do I work with this instruction manual? ............................. 1-1
1.3 Explanation of the safety instructions ......................................... 1-1
2 INITIAL OPERATION ........................................................ 2-1
3 RISKS AND SAFETY
OF HIGH-FREQUENCY SURGERY .................................. 3-1
3.1 Unintentional thermal tissue damage .......................................... 3-1
3.1.1 - due to HF leakage currents ....................................................... 3-1
3.1.2 - due to unintentional activation of an HF generator ................... 3-2
3.1.3 - due to inappropriate application ................................................ 3-3
3.1.4 - due to inappropriate or nonapplication of
the neutral electrode .................................................................... 3-3
3.1.5 - due to unsuitable and/or faulty accessories ............................... 3-4
3.1.6 - due to inattentiveness ................................................................ 3-5
3.1.7 - due to an output error ................................................................ 3-5
3.1.8 - due to the ignition of flammable liquids, gases
and/or vapors ............................................................................... 3-5
3.1.9 Unintentional burns due to hot electrodes ................................... 3-6
3.2 Electric shock .............................................................................. 3-6
3.3 Stimulation of nerves and muscles .............................................. 3-6
3.4 Cardiac pacemaker ...................................................................... 3-7
3.5 Danger of explosion .................................................................... 3-7
3.6 Interference with other electronic equipment .............................. 3-7
4 DESCRIPTION OF THE HIGH-FREQUENCY
SURGICAL UNIT ............................................................... 4-1
4.1 General description ..................................................................... 4-1
4.2 Description of the controls .......................................................... 4-4
1 Power switch .......................................................................................... 4-4
2 AUTO CUT function field ..................................................................... 4-5
3 AUTO COAG 1 function field ............................................................... 4-6
4 AUTO COAG 2 function field ............................................................... 4-7
5 AUTO BIPOLAR function field ............................................................ 4-8
6 Working with programs ......................................................................... 4-9
7 Connecting socket for neutral electrodes ............................................. 4-12
8 Connecting socket for the AUTO CUT and AUTO COAG 1
function fields ...................................................................................... 4-12
9 Connecting socket for the the AUTO CUT and AUTO COAG 2
function fields ...................................................................................... 4-12
10 Connecting socket for the AUTO BIPOLAR function field ................ 4-13
11 Safety field ........................................................................................... 4-13
12 Connecting socket for a dual-pedal footswitch .................................... 4-13
13 Connecting socket for a single-pedal footswitch ................................. 4-13
14 Terminal for potential equalization ...................................................... 4-14
15 Volume for acoustic signals ................................................................. 4-14
16 Loudspeaker for acoustic signals ......................................................... 4-14
17 Power connection ................................................................................. 4-14
18 Power fuses .......................................................................................... 4-14
4.3 Description of the safety features .............................................. 4-14

5 ERBOTOM ICC 350 Z ....................................................... 5-1
6 ERBOTOM ICC 350 T ....................................................... 6-1
7 ERBOTOM ICC 350 M....................................................... 7-1
8 ERBOTOM ICC 350 M-DOKU ........................................... 8-1
9 TECHNICAL DATA, SIGNALS, DIAGRAMS..................... 9-1
9.1 Technical data .............................................................................. 9-1
9.2 Visual and acoustic signals .......................................................... 9-5
9.3 Diagrams ..................................................................................... 9-6
10 INSTALLATION ............................................................... 10-1
11 CLEANING AND DISINFECTION OF THE UNIT ............ 11-1
12 PERFORMANCE CHECKS ............................................. 12-1
12.1 Automatic performance test after starting up the unit ............... 12-1
12.2 Automatic performance check during activation ...................... 12-1
12.3 Automatic error documentation ................................................. 12-2
Error list..................................................................................... 12-3
13 SAFETY CHECKS ........................................................... 13-1
14 MAINTENANCE, CARE, DISPOSAL .............................. 14-1
15 GUARANTEE .................................................................. 15-1
ADDRESSES

1-1
1 INTRODUCTION
1.1 Intended purpose of the ICC 350
The ICC 350 is a high-frequency surgical unit for cutting and coagulation. Due to its performance
features, it has universal applications. To meet the special requirements of surgical specialty
fields, the ICC 350 is available in several versions.
1.2 How do I work with this instruction manual?
Please read the instruction manual for the ICC 350. It applies to all versions of this high-frequency
surgical unit. If you have an ICC 350 Z, for example, please additionally read the chapter ICC
350 Z.
1.3 Explanation of the safety instructions
The WARNING safety instruction indicates a danger which can result in personal injury.
The CAUTION safety instruction indicates a danger which can result in property damage.
The IMPORTANT safety instruction indicates a danger which can cause functional failure of the
unit.

1-2

2-1
2 INITIAL OPERATION
Read carefully before initial operation of the unit.
In the development and production of this high-frequency surgical unit, the relevant, generally
recognized rules of technology, as well as the valid occupational safety and accident prevention
regulations have been taken into consideration. This ensures that patients, employees and third
parties are protected from dangers to life and health during intended application of the high-
frequency surgical unit, to the extent permitted by the type of application intended.
Initial operation
Before delivery, every high-frequency surgical unit is tested by the manufacturer in regard to its
function and safety. To ensure that the unit also functions safely after shipping and installation at
the operator’s site, the following points should be observed:
The operator should only operate the high-frequency surgical unit if the manufacturer or supplier
1. has subjected the unit to a performance test on site
2. has instructed the parties responsible for operation of the unit in handling of the unit by
means of the instruction manual.

2-2

3-1
3 RISKS AND SAFETY OF HIGH-FREQUENCY SURGERY
3.1 Unintentional thermal tissue damage
High-frequency surgery is associated in principle with various risks for the patient, the personnel
and surroundings. In order to avoid these risks in practice, the surgeon and his/her assistants
must recognize these risks and observe the appropriate rules for prevention of damage. In the
following, these risks and rules for prevention of damage are explained.
3.1.1 Unintentional thermal tissue damage due to HF leakage currents
During high-frequency surgery, the patient unavoidably conducts high-frequency electrical current
to ground potential. If the patient makes contact with electrically conductive objects during
high-frequency surgery, a high-frequency electrical current can result at the contact point between
the patient and this object, which can in turn cause thermal necroses. Not just objects made of
metal are electrically conductive objects, but also wet cloths.
WARNING
The patient must be insulated against electrically conductive objects during high-frequency
surgery. The black elastic table covers on operating tables demonstrate a certain electrical
conductivity for diverting electrical charges. Therefore they are never suitable for ensuring the
required insulation of the patient against metal parts of the operating table. For this reason, an
electrically insulating intermediate layer, for example dry cover cloths, must be laid between the
patient and this black operating table cover during the application of high-frequency surgery.
Fig.: Insulated positioning of the patient on the operating table
If it is possible for this intermediate layer to become wet during the operation, for example due
to perspiration, irrigation liquid, urine etc., wetting of these intermediate layers must be prevented
by a watertight sheet of plastic. Urine should be carried away via catheter.
Extremities lying against the trunk or skin-to-skin contact points should be insulated from
one another by laying dry cover cloths between them.
Do not apply ECG electrodes closer than 15 cm next to the operating field.
Needle electrodes or injection cannulae should not be used as ECG electrodes during
high-frequency surgery.
Electrically insulated
surface
Grounded
operating
table

3-2
3.1.2 Unintentional activation of an HF generator
Unintentional activation of an HF generator can lead to burns on the patient if the active electrode
hereby touches the patient directly or indirectly through electrically conductive objects or wet
cloths.
Unintentional activation of an HF generator can, for example, be caused by:
Unintentionally pressing a footswitch pedal
Unintentionally pressing a fingerswitch
Defective fingerswitches, footswitches or cables
Penetration of electrically conductive liquids (blood, amniotic fluid, urine, physiological
saline solution, irrigation fluids etc.) into fingerswitches or footswitches.
Errors within the high-frequency surgical unit
WARNING
To prevent burns on the patient due to unintentional activation of a high-frequency generator,
the following application rules should be heeded:
Never lay active electrodes onto or beside a patient in such a way that they can touch the
patient directly or indirectly through electrically conductive objects or wet cloths.
The lines to the active electrodes should be positioned in such a way that they touch
neither the patient nor other lines.
Always set the acoustic signal, which indicates the active status of the high-frequency
generator, so that it can be easily heard.
For operations in which the cutting or coagulation electrode unavoidably remains in contact
with the patient even in a nonactive condition, e.g. for endoscopic operations, particular
care is required. If such an electrode is unintentionally activated due to an error, this activated
electrode should then not be removed from the body without special supervision. When
removing the activated electrode from the patient’s body, burns can result on all areas
within the body which come into contact with the activated electrode. For this reason, in
case such errors occur, the power switch for the high-frequency surgical unit should be
switched off immediately before an attempt is made to remove the activated electrode
from the body.

3-3
3.1.3 Unintentional thermal tissue damage due to inappropriate application
Generally speaking, the bipolar coagulation technique should be applied in preference to the
monopolar coagulation technique. This particularly applies to coagulations on straight organs,
on which the high-frequency current flows over longer areas through diameters which are
approximately equal or become even smaller.
Fig.: Thermal damage of lateral tissue
The tissue is always first heated at places on the tissue where the diameter is smallest. If the HF current
flows through the same diameter (a) over longer distances, the tissue coagulates over this entire distance.
If the diameter of the tissue next to the application point of the coagulation electrode is smaller than at the
point of application, coagulation will also occur next to the application point (b).
WARNING
Always make certain that the HF current does not flow through thin tissue structures or vessels
with a small diameter.
3.1.4 Unintentional thermal tissue damage due to inappropriate or
nonapplication of the neutral electrode
With inappropriate or even nonapplication of the neutral electrode, there is a large risk of
unintentional thermal tissue damage both at the application point of the neutral electrode as well
as to other areas on the patient’s body.
The neutral electrode must be applied with its entire surface as closely and reliably as possible to
the operating field on the patient’s body.

3-4
WARNING
The effective contact surface, i.e. the electrical conductive value between the neutral electrode
and the patient must correspond to the HF capacity used, meaning the intensity of the HF current.
Here the effective contact surface means the surface of the neutral electrode which has electrically
conductive contact to the skin of the patient during high-frequency surgery.
Fig.: The neutral electrode must be applied at an appropriate location on the patient’s skin using the
entire contact surface available (a). If the neutral electrode has only partial contact to the patient’s skin (b),
there is a risk that burning will occur at this location
3.1.5 Unintentional thermal tissue damage due to unsuitable and/or faulty
accessories
It must be ensured that only accessories in perfect condition are used for high-frequency surgery.
Only accessories that are compatible or tested by the unit manufacturer must be used. This
applies both to the active electrodes including cable and plugs, as well as to the neutral electrodes
including cables and plugs.
When using an instrument with electric insulation, it is necessary to be certain that these insulations
are not overloaded and destroyed by overly high electric voltages. The electric output voltages
for the high-frequency surgical unit are indicated for the various cutting and coagulation modes
relative to the possible settings in this instruction manual. The electric strength of the instrument
insulation can be found in the technical data for the instruments or, in case of doubt, can be
requested from the manufacturer of the respective instrument.
WARNING
All insulation on electrodes, electrode holders, cables, plugs etc. must be in perfect condition.
a) b)

3-5
3.1.6 Unintentional thermal tissue damage due to inattentiveness
Like a scalpel, high-frequency surgery is always a potential source of danger if handled without
care.
WARNING
The cutting or coagulation electrodes should always be handled with care and laid aside in the
intervals between use so that neither the patient nor other persons can come in contact with the
electrodes.
Laying unused electrode handles or coagulation forceps on the patient, next to the patient or
within folds on the cover cloths is dangerous. Cases of burns on patients are known which were
caused by laying the coagulation forceps within folds on the cover cloths which penetrated
through the cloths into the patient’s skin and resulted in burns without being noticed.
3.1.7 Unintentional thermal tissue damage due to output error
The risk of unintentional thermal tissue damage is proportionate to the intensity and time limit
set on the unit for cutting or coagulation.
WARNING
The intensity for cutting or coagulation should only be set and only activated for as long as
necessary for the intended purpose.
An insufficient effect at a standard setting can, for example, be caused by poor attachment of the
neutral electrode, poor contact in the connectors, defective cables or electrically insulating tissue
remnants on the active electrode. This must be checked before setting at a higher power.
3.1.8 Unintentional thermal tissue damage due to the ignition of flammable
liquids, gases and/or vapors
During high-frequency surgery, electric sparks or arcs that can ignite flammable liquids, gases
or vapors occur at the active electrode.

3-6
WARNING
Make certain during high-frequency surgical operations that anesthetics, skin cleaning agents
and disinfectants are nonflammable. If their use is unavoidable, they must have completely
evaporated and the vapor must be removed from the area of spark formation before switching on
the high-frequency surgical unit.
Before application of high-frequency surgery in the gastro-intestinal tract, it must be ensured
that no flammable (endogenous) gases are present here. There is danger of explosion if flammable
gases are present. For this reason, these gases must be extracted and/or eliminated by flushing
out the affected lumen with CO2before using high-frequency surgery.
During transurethral resection (TUR), H2O molecules may dissociate into H2and O2in the arc
between the resection loop and the irrigation liquid. These gases may collect on the roof of the
urinary bladder as a highly explosive gas mixture. If resection is performed in this gas mixture,
dangerous explosions may occur.
3.1.9 Unintentional burns due to hot electrodes
Cutting and/or coagulation electrodes become hot during cutting and/or coagulation procedures
indirectly through the heated tissue and through the electric arc.
WARNING
Tissue can be unintentionally burnt immediately after cutting and/or coagulation procedures if
electrodes that are still hot touch the tissue. Attention must be especially paid to this during
endoscopic operations, such as during pelviscopic fallopian tube coagulation or during endoscopic
polypectomy.
3.2 Electric shock
An electric shock may occur if the high-frequency surgical unit delivers a too heavy low-frequency
current or if a too heavy low-frequency current flows through the patient into the high-frequency
surgical unit from another voltage source.
3.3 Stimulation of nerves and muscles
A known risk of high-frequency surgery is the unintentional electric stimulation of the patient’s
nerves and muscles. This stimulation can result from low-frequency electrical currents that are
caused either by low-frequency current sources or due to electrical arcs between an active electrode
and the patient’s tissue.
Electric alternating current with a frequency above 300 kHz is unable to stimulate nerves and
muscles.

3-7
During cutting procedures, forced coagulation and spray coagulation, the unavoidable electric
arcs between an active electrode and the tissue nevertheless have the effect that a portion of the
high-frequency alternating current is rectified, from which more or less strongly modulated,
low-frequency current components result which stimulate electrically stimulable structures such
as nerves and muscles.
This can result in more or less strong spasms or muscle contractions.
WARNING
When using high-frequency surgery on electrically stimulable structures, contractions of the
affected muscles must be taken into account. This can occur, for example, during endoscopic
operations in the urinary bladder in the vicinity of the obturator nerve and during operations in
the area of the facial nerve.
3.4 Cardiac pacemaker
For patients with implanted cardiac pacemakers or pacemaker electrodes, irreparable damage to
the pacemaker and disturbance of the pacemaker function, which can lead to ventricular fibrilation,
must be reckoned with.
3.5 Danger of explosion
High-frequency surgical units always generate sparks during operation on the active electrode.
For this reason, it is necessary to make certain during interventions that anesthetics, degreasers
and disinfectants are neither flammable nor explosive. They should at least have evaporated
completely before switching on the high-frequency surgical unit and be removed from the area
of spark formation.
3.6 Interference with other electronic equipment
High-frequency surgical units normally generate high-frequency electrical voltages and currents
which can interfere with other electronic equipment.
When installing or arranging sensitive electronic equipment in the operating room, this problem
should be taken into consideration. In principle, sensitive electronic equipment should be set up
as far as possible from the high-frequency surgical unit and particularly from the cables providing
HF current. In addition, the cables providing HF current, which act like broadcast antennas,
should not be unnecessarily long and should never be positioned parallel or too close to cables
from sensitive electronic equipment.
The unit has been fitted with a special generator in consideration of the disturbance of sensitive
electronic equipment, which generates a relatively low interference level as compared to
conventional high-frequency surgical units.

3-8

789 10International
Standard

This manual suits for next models
4
Table of contents
Other Erbe Medical Equipment manuals

Erbe
Erbe ICC 200 User manual

Erbe
Erbe 20134-004 Operating instructions

Erbe
Erbe VIO 300D ESU User manual
Erbe
Erbe 20321-040 User manual

Erbe
Erbe IES 2 User manual
Erbe
Erbe HybridKnife User manual

Erbe
Erbe 20193-008 Operating instructions
Erbe
Erbe ESM 2 User manual

Erbe
Erbe IES 3 User manual

Erbe
Erbe VIO 300 D User manual
Popular Medical Equipment manuals by other brands

InfraScan
InfraScan Infrascanner 2000 Operation manual

Handicare
Handicare LinidoSolutions LI2403.150 Series manual

burmeier
burmeier ECONOMIC II 51.0714.33 instruction manual

TASKA Prosthetics
TASKA Prosthetics TASKA Quick start user guide

videomed
videomed Truelink 4 Service manual

bort medical
bort medical DigiSoft quick guide