Trio SCF IFU Rev E 3/31/10 2:05 PM Page 3
Composite
C M Y CM MY CY CMY K
2 |QUESTIONS ABOUT TRIO? 1-888-335-6946
BSAFETY PRECAUTIONS AND IMPORTANT SAFEGUARDS
READ ALL DANGERS AND WARNINGS BEFORE USING
DANGER
To reduce the risk of serious or fatal injury from electrocution:
1. DO NOT place or store the Trio Electronic Nebulizer where it can
be in water or fall into a bathtub, sink, or other liquid. Do not place
or drop into water or other liquid. Do not use while bathing.
2. DO NOT reach for the Trio Electronic Nebulizer if it has fallen into
water or other liquid. Unplug immediately. Retrieve Trio Electronic
Nebulizer ONLY after it has been unplugged.
WARNING
1. The Trio Nebulizer Handset is for single patient use. DO NOT SHARE
your handset with any other people.
2. Read, understand and follow all warnings and instructions in the
Instructions For Use prior to using this device.
3. To reduce the risk of serious or fatal injury from electrocution,
fire, burns and to reduce the risk of damage and malfunction of
the unit:
a. DO NOT overload wall outlets or use extension cords.
b. Keep all electrical cords away from heated surfaces.
c. DO NOT spray liquids onto the housing of the Controller. Liquid
may cause damage to the electrical parts and could lead to a
malfunction. In the event that liquids enter the Controller,
call the number below for examination and repair.
d. DO NOT drop or insert any object into any opening on the Trio
Nebulizer System.
e. DO NOT operate where oxygen is being administered in a closed
environment such as an oxygen tent.
4. Always unplug this product immediately after using and
before cleaning.
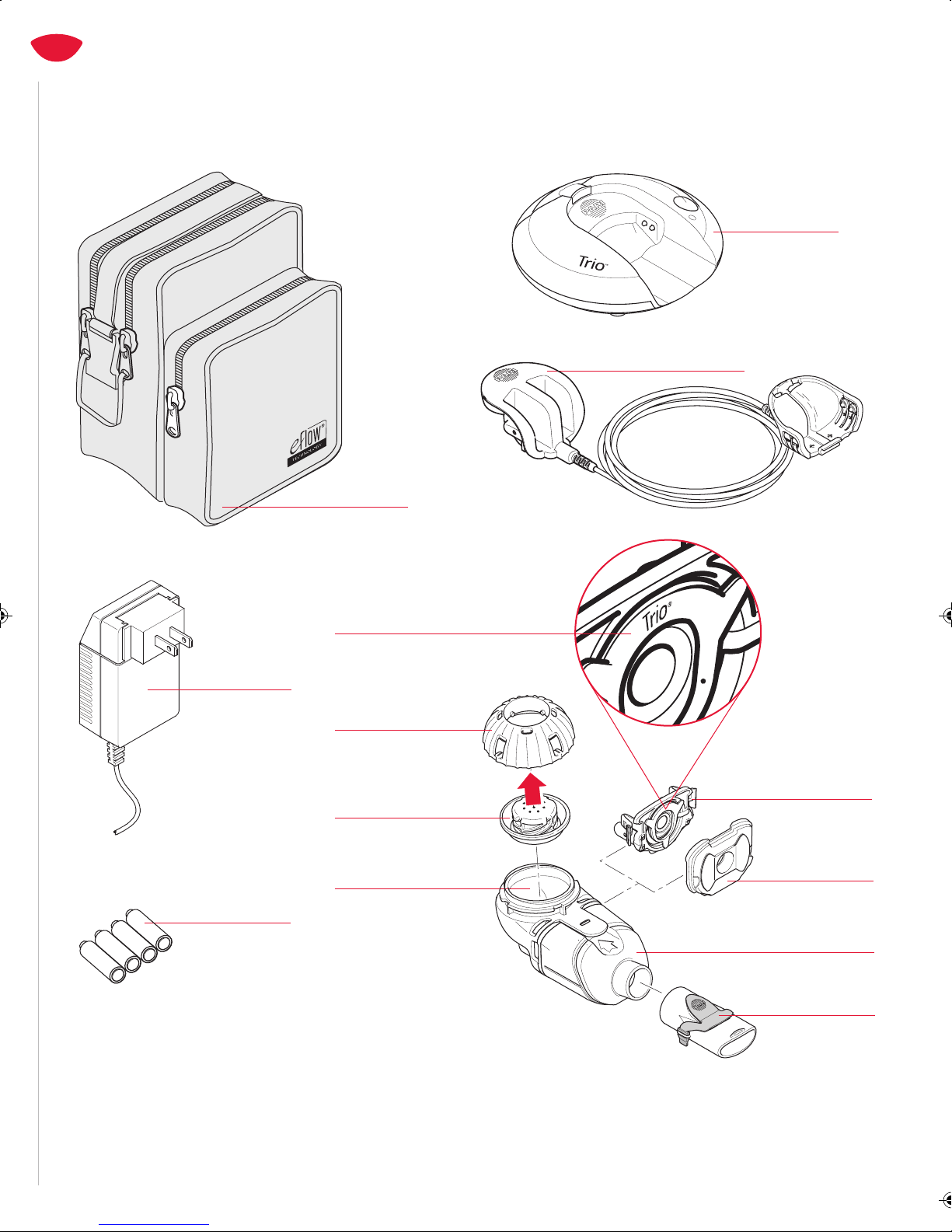
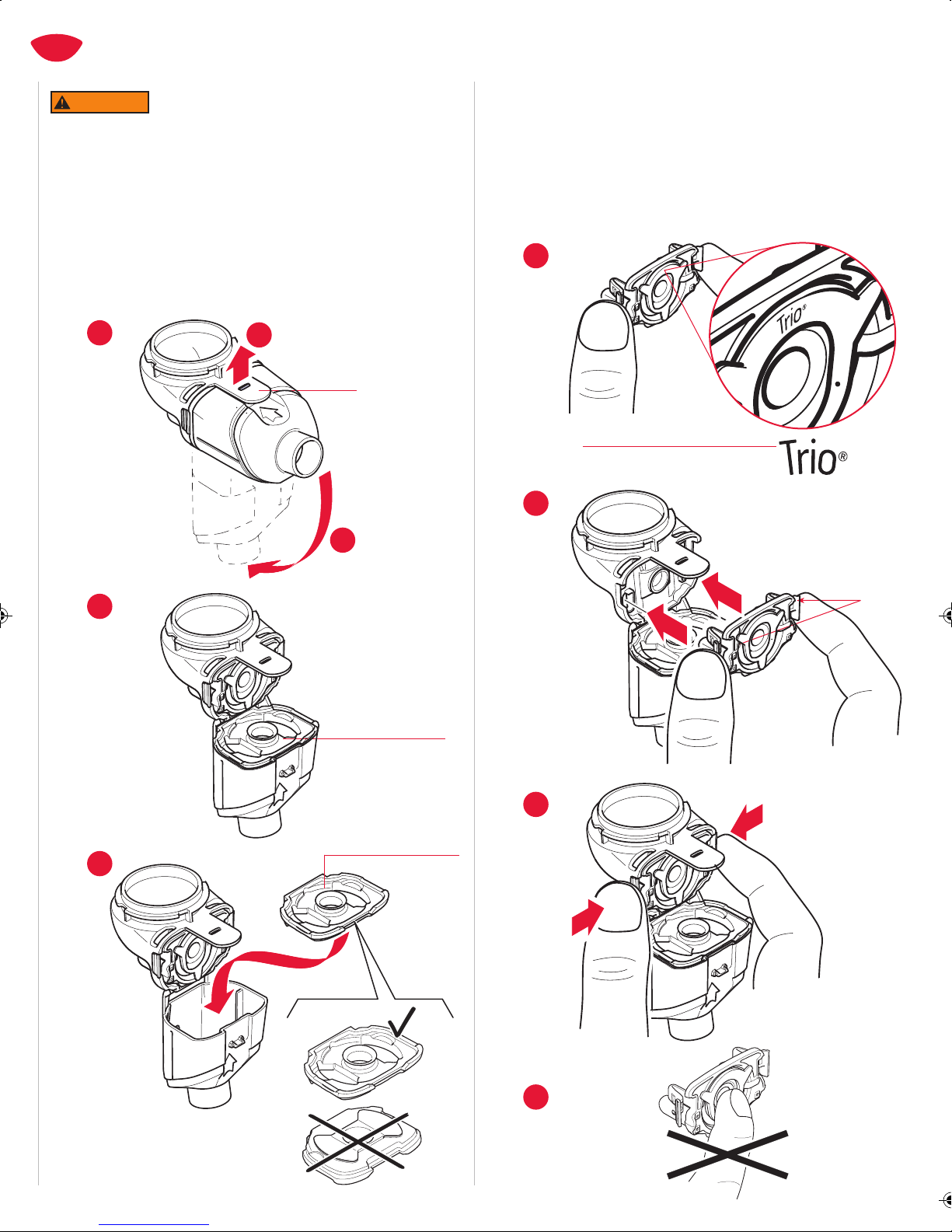
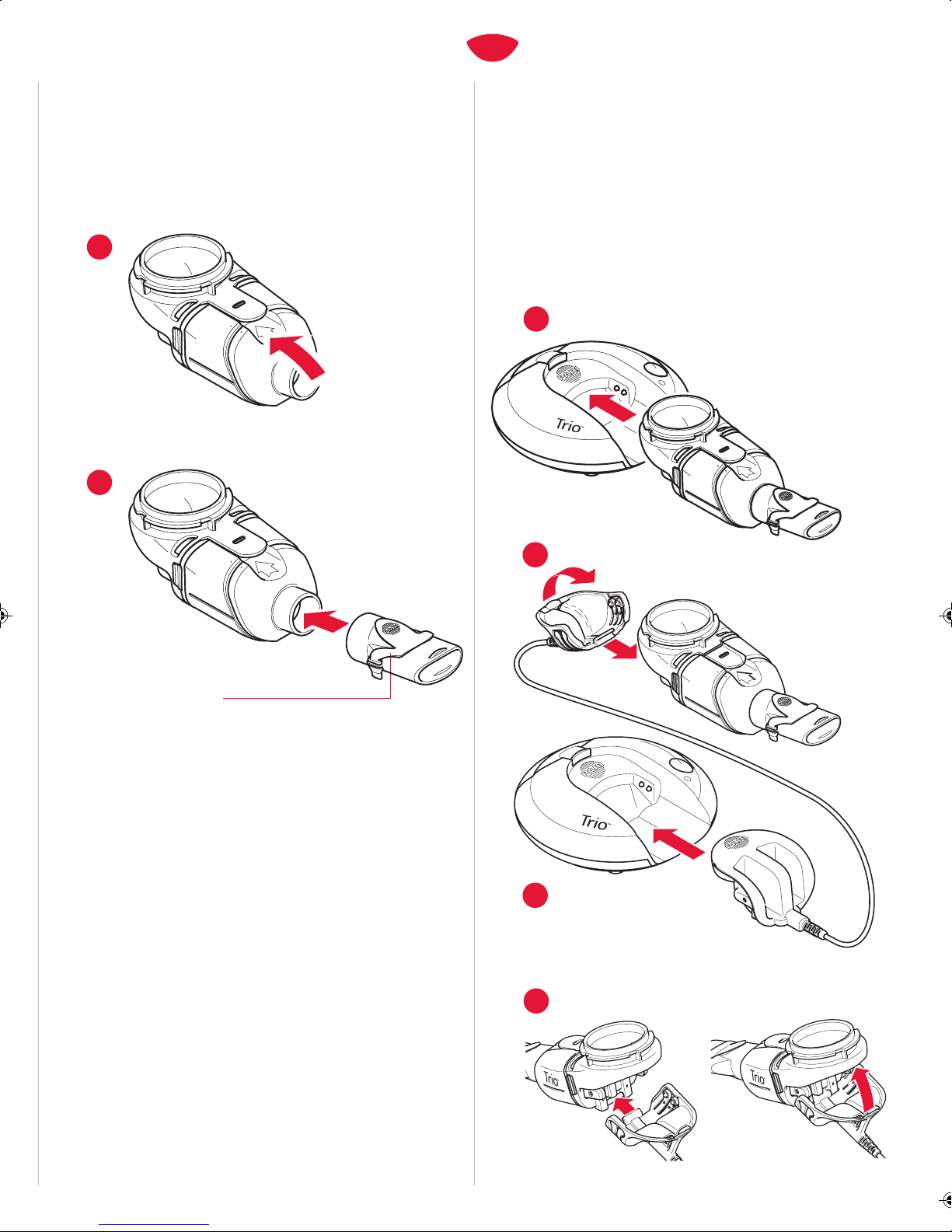
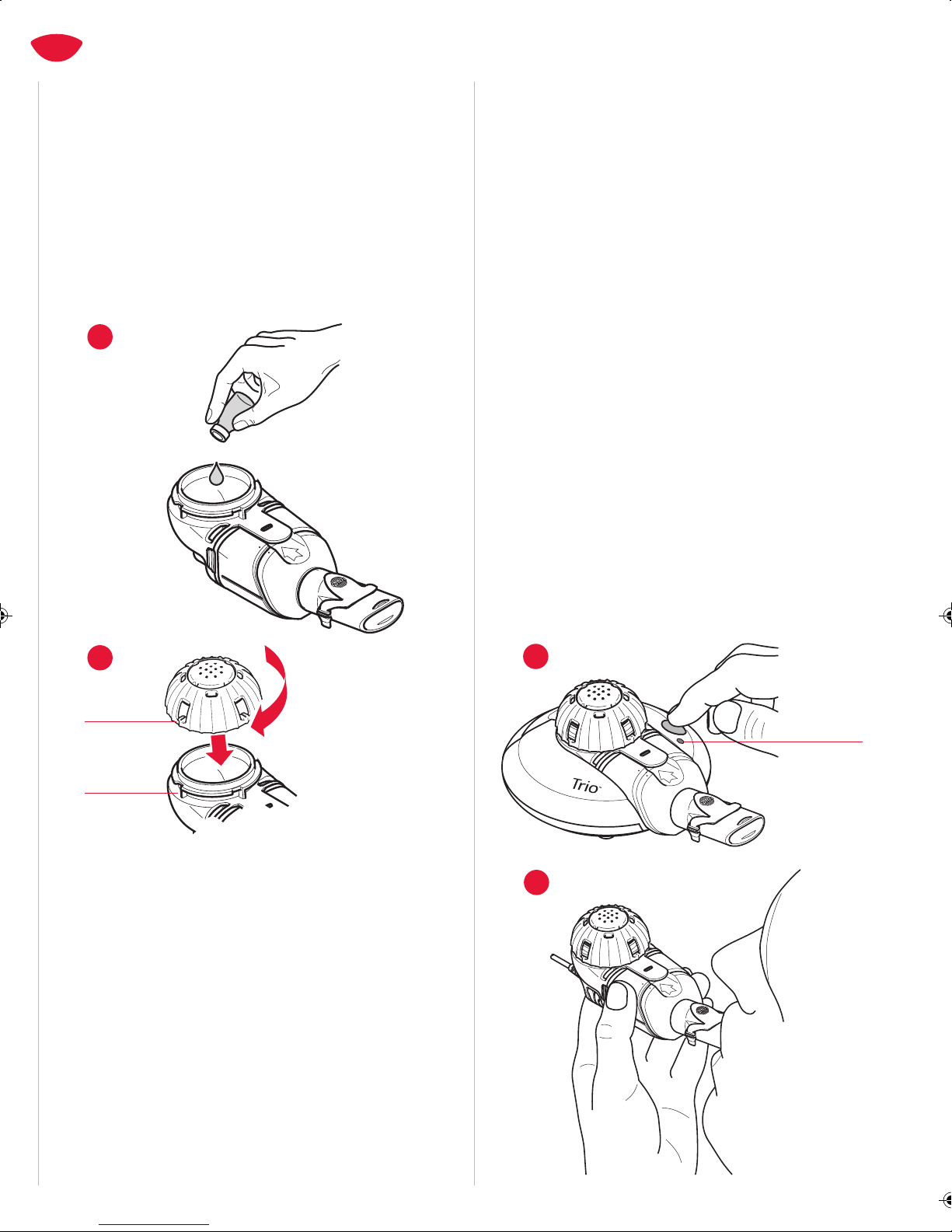
5. Before use, check your Trio Nebulizer Handset for proper assembly.
All parts must be connected and firmly in place. Use of an
improperly assembled Trio Electronic Nebulizer could diminish or
eliminate the effectiveness of the treatment.
6. Use ONLY adapters and accessories that are authorized for the
Trio Electronic Nebulizer. Use of unapproved adapters and
accessories can lead to improper treatment, injury, or damage to
the Controller.
7. NEVER operate the Controller if it is improperly or incompletely
assembled or damaged. Section G: Taking a treatment, shows
the alerts to these situations when the Trio Electronic Nebulizer
is improperly assembled, or might be damaged.
8. NEVER operate this product if:
a. it has damaged cords or plugs,
b. it is not working properly,
c. it has been dropped or damaged, or
d. it has been exposed to any liquids inside the Controller.
9. To reduce the risk of an infection, illness, or injury from
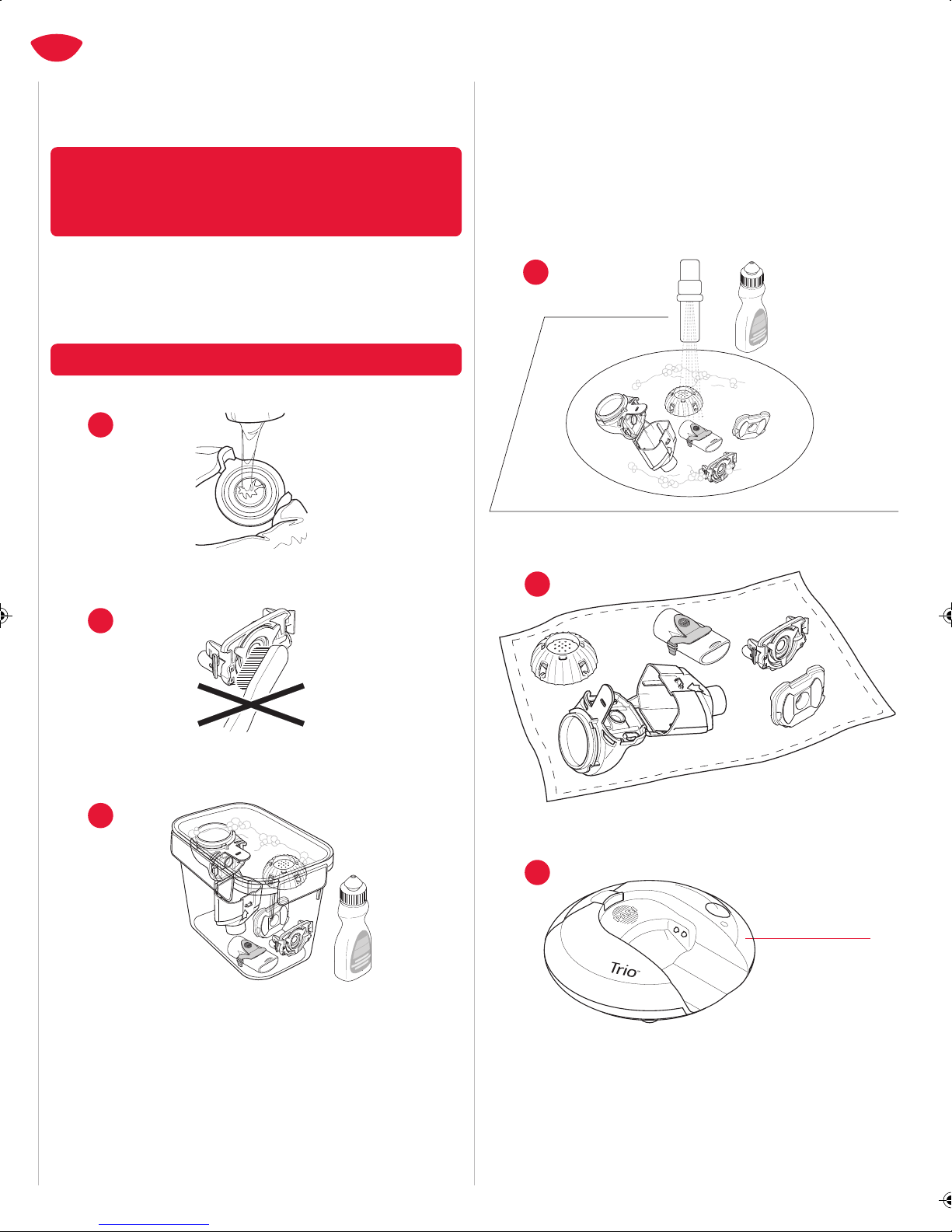
contamination, clean and dry all parts of the Trio Nebulizer Handset
after each treatment. Follow the instructions in Section H to clean
the Trio Nebulizer Handset properly.
10. Cleaning the Trio Nebulizer Handset properly will help prevent the
Aerosol Head from clogging. If the Aerosol Head becomes clogged,
the aerosol mist will be reduced or stopped, which may make the
treatment ineffective. If clogging occurs, use the instructions in
Section H to clean the Aerosol Head.
11. Cleaning the Trio Nebulizer Handset only removes the medication.
To reduce the risk of serious or fatal illness caused by
contamination of the Trio Nebulizer Handset, YOU MUST ALSO
DISINFECT THE TRIO NEBULIZER HANDSET AT THE END OF EVERY
TREATMENT DAY. See Section I for disinfection instructions.
12. This product contains small parts that may present a choking
hazard to small children. The Nebulizer Connection Cord also
presents a strangulation hazard.
13. DO NOT LEAVE CHILDREN UNATTENDED DURING TREATMENT. ALWAYS
USE CLOSE ADULT SUPERVISION WHEN ADMINISTERING A
TREATMENT TO A CHILD.
14. Close supervision is necessary when this product is used by or
near children or the physically or mentally impaired.
15. DO NOT use your Trio Electronic Nebulizer while driving or in any
situation which takes away your full attention.
16. If the Trio Electronic Nebulizer has been damaged or is not operating
properly, call the number below for details. If you suspect that the
Trio Electronic Nebulizer has been damaged or is not working
correctly, run the Functionality Test described in Section J.
17. DO NOT disassemble the Controller at any time. There are no user
serviceable parts inside the Controller. Call the number below for
all service needs.